The year in cardiovascular medicine 2020: heart failure and cardiomyopathies
█ Current opinion
Authors:
Héctor Bueno1,2,3,4,*, Brenda Moura5,6, Patrizio Lancellotti7,8, and
Johann Bauersachs9
Corresponding Author:
1Multidisciplinary Translational Cardiovascular Research Group. Centro Nacional de Investigaciones Cardiovasculares (CNIC), Melchor Fernández Almagro, 3, Madrid 28029, Spain; 2Cardiology Department, Hospital Universitario 12 de Octubre and Instituto de Investigación Sanitaria Hospital, 12 de Octubre (imas12), Madrid, Spain; 3Centro de Investigación Biomédica en Red Enfermedades Cardiovaculares (CIBERCV), Madrid, Spain; 4Facultad de Medicina, Universidad Complutense de Madrid, Plaza de Ramón y Cajal, s/n, 28040 Madrid, Spain; 5Cardiology Department, Military Hospital, Av. da Boavista S/N, 4050-115 Porto, Portugal; 6CINTESIS – Center for Health Technology and Services Research, R. Dr. Plácido da Costa, 4200-450 Porto, Portugal 7Department of Cardiology, CHU SartTilman, University of Liège Hospital, GIGA Cardiovascular Sciences, Avenue de L’Hôpital 1, 4000 Liège, Belgium; 8Cardiology Departments, Gruppo Villa Maria Care and Research, Maria Cecilia Hospital, Cotignola Bari, Italy and Via Corriera, 1, 48033 Cotignola RA, Italy and Anthea Hospital, Via Camillo Rosalba, 35/37, 70124 Bari BA, Italy; and 9Department of Cardiology and Angiology, Hannover Medical School, Carl-Neuberg-Str. 1, 30625 Hannover, Germany
Summary
Heart failure (HF) prevalence remains high worldwide with significant sex-related and regional differences in its presentation, management, and outcomes. In 2020, advances in biomarkers and imaging techniques were reported for the diagnosis and prognosis of diastolic dysfunction, HF with preserved ejection fraction or monitoring cardiotoxicity; a new definition of HF with recovered left ventricular ejection fraction (LVEF) was released.
Benefits of renin–angiotensin–aldosterone system inhibitors and β-blockers may extend to patients with an LVEF up to 55%. Sacubitril–valsartan improved LV remodeling, biomarker levels, and rates of sudden cardiac death.
Two studies investigating the sodium-glucose cotransporter 2 inhibitors empagliflozin and sotagliflozin in patients with HF were reported: the EMPEROR-Reduced trial in patients with HF with reduced EF with or without type 2 diabetes (T2DM) demonstrated a significant reduction in cardiovascular (CV) death and HF hospitalisations (HFH). In patients with T2DM and HF across the whole EF spectrum after a recent HFH, the SOLOIST trial showed a reduction in the primary endpoint of CV deaths, total HFH, and urgent visits for HF. In addition, in patients with kidney disease with or without diabetes mellitus (DAPA-CKD), dapagliflozin prevented the deterioration of renal function. Two novel drugs, the activator of soluble guanylate cyclase vericiguat and the myosin activator omecamtiv-mecarbil, in the large outcome trials VICTORIA and GALACTIC-HF predominantly reduced HFH in high-risk patients with worsening HF. In the AFFIRM-AHF trial, intravenous ferric carboxymaltose reduced HFH in patients with iron deficiency after an HF decompensation.
Year 2020 will be remembered as the year of coronavirus disease of 2019 (COVID-19). The pandemia caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused a massive impact on global health and economy. When this article is published, >80 million people will have been infected and >1.75 million will have died of the disease. Many others will have died or worsen of their diseases, many with cardiovascular (CV) disease, as an indirect effect of the fear to seek assistance or the collapse of healthcare systems. Yet, advances in science and medical care continued developing during the year. This article reviews important advances in the field of heart failure (HF) presented in 2020.
ISSUE: CARDIOLOGIA HUNGARICA | 2021 | VOLUME 51, ISSUE 2
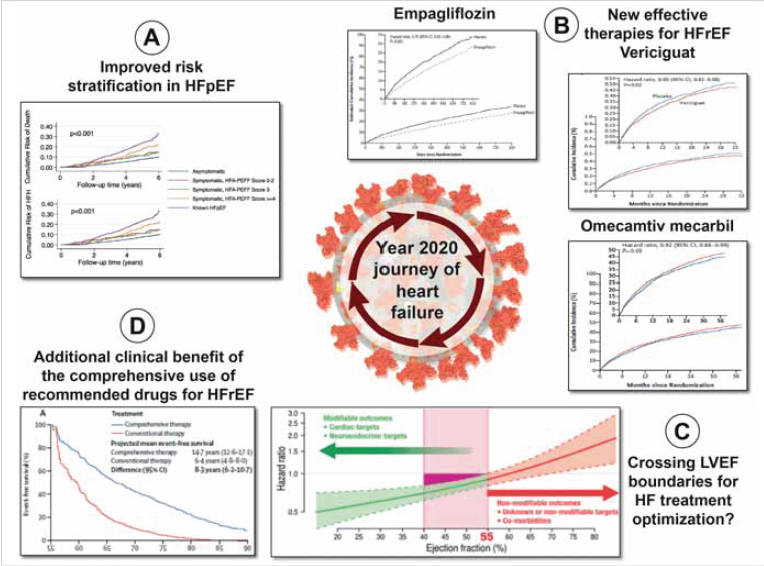
Graphical Abstract: During year 2020, we learned new options to better stratify patients with heart failure and preserved left ventricular ejection fraction (HFpEF) (A), the clinical benefit of three new drugs to improve prognosis of patient with heart failure and reduced left ventricular ejection fraction (HFrEF): empagliflozin, vericiguat and omecamtiv mecarbil (B), the potential benefit of a broader utilization of recommended drugs for HFrEF in patients with left ventricular ejection fraction higher than 40% (C), and the potential added clinical benefit of a comprehensive use of recommended drugs for HFrEF (D) in a year marked by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic (central cartoon). Reprinted or adapted from: (A) Selvaraj et al.,(23) (B) Packer et al., (115) Armstrong et al., (126) and Teerlink et al., (132) (C) Böhmet al., (100) (D) Vaduganathan et al. (139)
From Bueno et al. European Heart Journal (2021) 42, 657–670. doi:10.1093/eurheartj/ehaa1061, by permission of Oxford University Press on behalf of the European Society of Cardiology
Epidemiology
More than 64 million people are living with HF in the world, with an estimated prevalence of 1–2% among adults in developed countries, most often with several comorbidities (Figure 1) (1). The incidence of HF may be stabilizing globally, with decreases in higher-income countries (2) but increases in lower-income countries, and a shift towards HF with preserved ejection fraction (HFpEF) and increasing due to population ageing and the increase in obesity (1). Age, traditional risk factors for HF, a sedentary lifestyle, and social deprivation are associated with incident HF (3). Actually, lifestyle and social determinants of health are attracting more attention in the epidemiology and care of patients with HF (4). In patients with new-onset HF, the most common first events are cardiac events (36%), recurrent HF (28%), and death (29%) (5).
Prevalence of heart failure in different world regions as estimated from population-based studies. Reprinted from Groenewegen et al. (1).
Non-traditional risk factors, such as pacemaker implantation may play a role in the development of HF: within the first 2 years after implantation in patients without known HF, the incidence of fatal and non-fatal HF is 10.6%, six times higher than for age- and gender-matched individuals without HF and pacemaker (6).
Mortality rates of HF seem to be declining less rapidly than previously in the general population (1). Among patients with cardiac resynchronization therapy (CRT), a gradual decrease in sudden cardiac death risk has been observed since the early 2000s (7) with implications for the role of implantable defibrillators and the design of comprehensive HF care models.
Significant regional differences in the management of acute HF have been identified, including timing and types of treatments used (8), and rates and time trends of readmission (2, 9, 10). However, the importance of distinguishing worsening/chronic HF from new-onset HF in patients with first hospitalization has been highlighted, as patients with worsening/chronic HF have a significantly greater comorbidity burden and higher adjusted risks of mortality and HF readmission (10, 11).
Clinical aspects
Diagnostics and risk stratification
Imaging
Imaging is pivotal in the diagnosis and risk stratification of patients with HF. The European Society of Cardiology (ESC) Heart Failure Association (HFA) has recently highlighted in a position statement the central role of full echocardiographic examination in patients admitted for acute heart failure (AHF) (12). Once the patient is stabilized, the added value of routine cardiac magnetic resonance (CMR) over echocardiography alone to help diagnose the causes of HF not related to ischaemic heart disease has been questioned (13). Selective rather than routine CMR for identifying specific HF aetiologies is more cost effective. Noteworthy CMR could serve to better define HFpEF phenotypes and to select patient specific therapies, such as MRA may be for HFpEF patients with myocardial fibrosis (14–17). The diagnosis of HFpEF remains challenging especially in patients with coexisting conditions that account for dyspnoea. Diastolic dysfunction, left atrial enlargement, elevated left atrial pressure, and pulmonary hypertension are common in these patients (18, 19). The 2016 diastolic dysfunction grading algorithm proposed by the European Association of Cardiovascular Imaging has shown improved prognostic value compared to the 2009 one (20).
However, the high number of patients with doubtful classification renders clinical decision making challenging (21). The analysis of LA mechanics, LA strain, and left ventricular (LV) global longitudinal strain (22) allows to better classify the degree of diastolic dysfunction and improves individual risk stratification. Two algorithms (H2FPEF and ESC HFA-PEFF) may facilitate HFpEF diagnosis. These two scores have equivalent predictive power of incident HF hospitalization or death among patients without a clinical diagnosis of HF (23). Although LV ejection fraction (LVEF) is key for HF classification, it remains a crude estimate of LV function. Intriguingly, 17% of patients with initially preserved LV systolic function show a decrease in LVEF below 40% at 6 months follow-up, which is associated with more cardiac events (24). Parameters of LV mechanics (LV strain, multilayer strain and myocardial work) provide incremental prognostic information over LVEF (22, 25). The benefit of treatment [i.e. sacubitril/valsartan (SV)] on LV remodelling is also better captured by LV strain (26). Myocardial mechanics is linked to coronary microvascular dysfunction in patients with hypertensive HF (27, 28). In AHF, cardiac sympathetic nerve dysfunction, as evaluated by 123I-metaiodobenzylguanidine imaging, is associated with poor outcome irrespective of LVEF (29).
Biomarkers
Biomarkers are key for diagnosis and prognostic evaluation in patients with HF. Circulating biomarkers related to extracellular matrix regulation were abnormal in patients with HFpEF, displayed prognostic value, and were influenced favourably by SV in PARAGON-HF (30). In HF with reduced LVEF (HFrEF), absolute NT-proBNP, hs-TnT, and sST2 levels predict outcomes independent of age, sex, and LVEF category (31). Differential circulating levels of biomarkers associated with ageing in patients with HF have been reported, with increasing levels of proteins associated with extracellular matrix organization, inflammatory processes, and tumour cell regulation and lower expression of tumour proliferation functions (32).
In AHF, a specific challenge is to identify infection as a trigger of AHF. Procalcitonin (PCT) has emerged as an alternative for C-reactive protein in diagnosing bacterial infection. In a recent randomized, multicentre, open study, a strategy of PCT-guided initiation of antibiotic therapy was more effective than standard care in improving clinical outcomes (33). Omics phenotyping is likely the next frontier to unravel disease mechanisms and heterogeneity (34). As a recent example, incorporating a panel of three metabolite-based biomarkers into a risk score improved the prognostic utility of NT-proBNP by predicting long-term CV death (35).
Heart failure during the COVID-19 pandemic
The role of the angiotensin-converting enzyme (ACE) receptor 2 in the infection of human cells by SARS-CoV-2 and in the pathophysiology of COVID-19 (36), and the poor prognosis of cardiac patients with COVID-19 (37) raised the concern of a potential deleterious effect of the treatment with ACE inhibitors and angiotensin receptor blockers (ARB). These drugs may either decrease acute lung damage, prevent angiotensin-II-mediated pulmonary inflammation or increase the SARS-CoV-2 pulmonary damage by the up-regulation of ACE2 receptors (38, 39). Observational studies refuted the hypothesis of a deleterious effect of ACEI/ARB (40–43). The BRACE CORONA trial found no worse outcomes in patients with COVID-19 allocated to continuation or interruption of their chronic ACEI/ARB treatment (presented at the ESC Congress, data not published).
The incidence of AHF or decompensation of chronic HF among patients with Covid-19 is high and with poor prognosis (44). Indirect effects of the pandemic included the reduction in HF hospitalizations during local outbreaks (45–47) with increases in their hospital mortality (45, 47) and major challenges for the management and Follow-up of HF patients, and the conduct of clinical trials. Recommendations to overcome these challenges have been released (48–50).
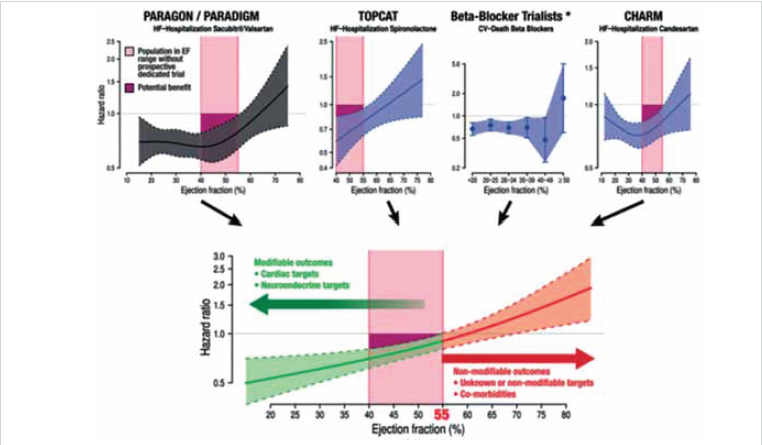
Figure 2. Results from different trials testing a number of drugs commonly used to treat heart failure, pointing to an extended benefit up to a left ventricular ejection fraction of 55%. For patients with left ventricular ejection fraction >55%, a population group usually presenting several comorbidities, there is still no evidence of a drug improving prognosis. Reprinted from Böhmet al. (100)
From Bueno et al. European Heart Journal (2021) 42, 657–670. doi:10.1093/eurheartj/ehaa1061, by permission of Oxford University Press on behalf of the European Society of Cardiology
Sex and heart failure
Women account for half of patients with HF with a lower incidence rate until the age of 75 years, a higher proportion of HFpEF, probably related to the higher prevalence of obesity and diabetes mellitus (1). Women with HF present a greater symptom burden and poorer quality of life as compared with men (51). Significant sex-related differences have been described in Europe in the management of acute and chronic HF (8, 52) including a lower use of guideline-directed medical therapies – which seem to be mostly explained by older age and comorbidity rather than by sex itself – with lower crude rates of death and HF hospitalization in women.
The lack of sex-related differences in the clinical effect of HF therapies (53, 54) does not justify these differences, although the possibility has been suggested that women with HF might benefit from treatment to a higher level of LVEF than previously considered (54). A different perspective of the gender gap in HF is the lower proportion of female authors in HF practice guidelines and trials, ranging between 11% and 24% only, with modest increases over time in European and US guidelines references but not in HF trials. Importantly, HF trials with a woman first or senior author are associated with a higher proportion of enrolled female participants (55).
Comorbidities
Comorbidities are important because they impact the clinical presentation, management, and outcomes of HF patients. The burden of comorbidities is higher in older patients, women and those with HFpEF (56–58), which are often ignored (59). Particularly relevant conditions in HF patients include atrial fibrillation (60) which has complex interrelations with HF needing more research (61, 62).
One example is the lack of increase in mortality risk associated with elevated heart rate in patients with HFrEF and atrial fibrillation, as compared to sinus rhythm (50, 63). Renal disease is one other, with renal function often changing during the course of the disease or as a response to HF therapies. Clinical responses, including worsening renal function and pseudo-worsening renal function, and their pathophysiological correlates, i.e. tubular function (diuretic response) beyond estimated glomerular filtration rate (eGFR), need to be understood to be properly managed, adapting therapies to the changing situation (64, 65).
Specific situations
Acute heart failure
In patients with acute HFrEF, istaroxime, an inhibitor of the sarcolemmal Na+/K+ pump activating the SERCA2a pump, improved cardiac function without major adverse effects in a small mechanistic trial (66). Cimlanod, a nitroxyl donor infused over 48 h, was reasonably well tolerated at a lower dose whereas higher doses caused unacceptable hypotension. There was improvement of NT-ProBNP but not on dyspnoea (presented at HFA Discoveries, data not published). A number of position papers have summarized the role of imaging (12) or the management of AHF in specific situations, such as acute coronary syndromes (67) or atrial fibrillation (68).
Cardiogenic shock
While its incidence seems to be decreasing, cardiogenic shock still conveys a high mortality risk (69). A new clinical classification (70) and two position papers (71, 72) on cardiogenic shock have been published this year. The SWEdish evaluation of left Ventricular Assist Device (SweVAD) will examine the impact of mechanical circulatory support vs. guideline-directed medical therapy on survival in a population of AHF patients ineligible for heart transplant (73).
Peripartum cardiomyopathy
Peripartum cardiomyopathy (PPCM) is the first cause of HF in women during/after pregnancy (74–76). The ESC EORP registry on PPCM enrolled >700 women with this condition from 49 countries. It showed that PPCM affects women from any region or ethnicity. Within 6 months after diagnosis, the average rates of maternal mortality, readmission, and neonatal mortality were, respectively, 6%, 10%, and 5%, with marked regional variations. Recovery of LVEF occurred in 46% of women (77). The management of these patients is reviewed in a recent paper (78).
HF with recovered left ventricular ejection fraction
This year, a working definition of HF with recovered left ventricular ejection fraction (HFrecEF) has been proposed. This includes:
(i) documentation of a decreased LVEF < 40% at baseline; (ii) ≥10% absolute improvement in LVEF; and (iii) a second measurement of LVEF >40% (79).
Reverse LV remodelling is associated with improved myocyte and LV chamber contractility and better clinical outcomes. However, a significant proportion of patients with HFrecEF develop recurrences of LV dysfunction and HF. Despite improvements in structural and functional abnormalities, many of the multilevel molecular changes occurring during LV remodelling remain dysregulated in reverse remodelled hearts. Therefore, guideline-directed medical and device therapy for patients with HFrecEF should be continued indefinitely with close clinical follow-up (79).
HF in cancer patients
The role of CV imaging in cancer patients receiving cardiotoxic therapies has been highlighted in a position statement by the HFA (12) and in the European Society for Medical Oncology guidelines (80). The role of focus echocardiography (81) and CMR (82) has also been recently discussed. In daily practice, caution should, however, be given if using late gadolinium enhancement or qualitative T2-weighted STIR imaging-only approach for the exclusion of checkpoint inhibitor-associated myocarditis (83). Imaging is cornerstone for monitoring cardiotoxicity and identifying subtle impairment of myocardial function occurring prior crossing the traditionally defined threshold of LV systolic dysfunction (LVEF < 50%) (84, 85).
Right ventricular dysfunction (RVD)
RV and right atrium dysfunction contribute to HFpEF pathophysiology. Also, RV dysfunction (lower RV systolic velocity and RV fractional area change) and impairment in RV-pulmonary artery coupling are more frequently found in HFpEF patients developing acute lung congestion with exercise (86). Activation of the endothelin and adrenomedullin neurohormonal pathways is associated with pulmonary haemodynamic derangements, reduced RV functional reserve, reduced cardiac output, and more severe impairment of peak VO2 in HFpEF patients (87). The most common causes of RVD are left-sided heart diseases (46%), pulmonary thromboembolic disease (18%), chronic lung disease/hypoxia (17%), and pulmonary arterial hypertension (11%). Average 1-year mortality in patients with RVD is high (>40%), highest among chronic lung disease patients (88). The presence of RVD at CRT implantation predicts worsening LV remodelling and survival (89).
Pharmacotherapies
Angiotensin receptor–neprilysin inhibitors (paragon, paradigm, parallax)
Angiotensin receptor–neprilysin inhibitor (ARNI) showed, in a sub-analysis of PARADIGM-HF, a reduction in sudden cardiac death risk regardless of the use of implantable cardiac defibrillators (90). Reduction in ventricular volumes and increase in LVEF have been observed with standard echocardiography in patients after 6 months on SV, but improvement in global longitudinal strain is apparent after 3 months (26). In a small cohort of patients with end stage renal disease, SV showed efficacy and safety (91). The LIFE Trial, comparing SV to valsartan in NYHA Class IV HFREF patients, although prematurely interrupted because of the COVID 19 pandemia, will still provide information about ARNI as a treatment option for advanced HF patients (92).
The PARALLAX trial tested the efficacy of SV vs. optimal individualised background therapy in HFpEF patients and found a reduction in NT-proBNP from baseline to 12 weeks but no effect on six-minute walk distance from baseline to 24 weeks (presented at ESC 2020 – data not published). In the PARAGON Trial in patients with HFpEF, SV did not result in a lower rate of total hospitalizations for HF and death. Of the 12 pre-specified subgroup analyses, sex and LVEF appeared to modify the effect of SV vs. valsartan on the primary composite outcome. Although no benefit was apparent in men, there was a significant reduction in HF hospitalizations in women (93). Also, patients seemed to derive more benefit from SV when started early after hospitalization (94). Baseline and mean achieved systolic blood pressure of 120–129 mm Hg identified the lowest risk HFpEF patients, but the blood pressure-lowering effects of SV did not account for its effects on outcomes, regardless of sex (95). Compared with valsartan, SV reduced the risk of renal events and slowed the decline in estimated glomerular filtration rate (96).
Reduction in serum uric acid was also associated with improved outcomes (97). A meta-analysis assessing the efficacy of different renin–angiotensin–aldosterone system (RAAS) antagonists in clinical trials performed in HFpEF patients (PEP-CHF, CHARM-preserved, I-PRESERVE, TOPCAT, PARAGON-HF) showed no statistical difference in all-cause and CV mortality among RAAS antagonists and placebo, but a significantly decreased risk in HF hospitalizations in patients allocated to receive ARNI compared with controls (OR: 0.73, 95% CI: 0.61–0.87) and ARB (OR: 0.80, 95% CI: 0.71–0.91) (98).
A patient-level data analysis from the PARADIGM-HF and PARAGON-HF trials (SV vs. enalapril in HFrEF and SV vs. valsartan in HFpEF, respectively), and the CHARM-Alternative and CHARM-Preserved trials (candesartan vs. placebo) showed that, compared with RAAS inhibitors, SV improved outcomes across the range of LVEF, with a risk reduction (RR) of 0.54 [95% confidence interval (CI) 0.45–0.65] for the recurrent primary endpoint compared with putative placebo (P < 0.001). Treatment benefits were robust in patients with LVEF < 60%, but not in those with LVEF > 60% (99). These results are in line with prior post hoc analyses from the TOPCAT study and β-blocker trials suggesting that the cut-off of LVEF for a beneficial treatment effects is ~55%. These analyses show that in the sparsely studied population of patients with an LVEF of 40–55%, several HF treatments might provide benefit (Figure 2) (100).
Results from different trials testing a number of drugs commonly used to treat heart failure, pointing to an extended benefit up to a left ventricular ejection fraction of 55%. For patients with left ventricular ejection fraction >55%, a population group usually presenting several comorbidities, there is still no evidence of a drug improving prognosis. Reprinted from Böhm et al. (100).
Sodium-glucose cotransporter 2 inhibitors (EMPEROR-Reduced, DAPA-HF, SOLOIST, VERTIS, SUGAR-DM-HF, EMPA-TROPISM [ATRU-4])
In patients with type 2 diabetes, the sodium-glucose cotransporter 2 (SGLT-2) inhibitors empagliflozin and dapagliflozin reduce the risk of HF hospitalization regardless of baseline CV risk or history of HF (101, 102). In The VERTIS trial, ertugliflozin did neither significantly reduce CV events, nor the combined endpoint of CV death/HF hospitalization (103) but reduced HF hospitalizations (104).
In patients with HFrEF, DAPA-HF has demonstrated a significant reduction in CV mortality and HF events (105, 106). This robust effect was analysed in more detail in several seminal papers published in 2020. The benefit of dapagliflozin was independent of the diabetes status, occurring across all levels of HbA1C (107), as well as of baseline renal function or blood pressure, patient age, or background HF therapy (108–111). Dapagliflozin improved symptoms, physical function, and quality of life (112) and was shown to be a cost-effective treatment for HFrEF in the UK, German, and Spanish healthcare systems (113). Dapagliflozin also reduces the rate of decline in renal function in HFrEF patients (111) as well as in patients with chronic kidney disease, as shown in the DAPA-CKD trial, where treatment with dapagliflozin reduced the risk of worsening renal function, end-stage kidney disease, or death. This protective effect was observed in patients with or without diabetes (111, 114).
Empagliflozin also showed marked beneficial effects in HFrEF patients independently from diabetes status (Figure 3), with a significant reduction in the primary composite endpoint of CV death and HF events (hazard ratio – HR), 0.75; 95% CI: 0.65–0.86; P < 0.001), the secondary endpoints of total HF hospitalizations (HR: 0.70; 95% CI: 0.58–0.85; P < 0.001), the annual rate of decline in the estimated glomerular filtration rate (−0.55 vs. −2.28 mL/min/1.73 m2 of body-surface area per year, P < 0.001), the risk of serious renal outcomes (115), and the risk and total number of inpatient and outpatient worsening HF events, which starts early after the initiation of treatment and remains during the duration of treatment (116). These beneficial effects were also observed to a similar extent in patients pre-treated with ARNI (117) and were independent of baseline diabetes status and across the continuum of HbA1c (118), and in patients with and without CKD and regardless of the severity of kidney impairment at baseline (119).
Primary outcome results from the EMPEROR REDUCED (top), VICTORIA (lower left), and GALACTIC (lower right) trials, testing empagliflozin, vericiguat, and omecamtiv mecarbil, respectively, in patients with heart failure with reduced left ventricular ejection fraction. Reprinted from Packer et al. (115), Armstrong et al. (126) and Teerlink et al. (132).
In the SUGAR-DM-HF study, empagliflozin reduced LV volumes measured by CV magnetic resonance in patients with HFrEF and type 2 diabetes or prediabetes (120). The mechanistic trial EMPA-TROPISM (ATRU-4) showed the beneficial effect of empagliflozin in improving LV volumes, LV mass, LV systolic function, functional capacity, and quality of life in non-diabetic patients with HFrEF (121) (ref). Taken the evidence together, SGLT-2 inhibitors reduce all-cause and CV mortality and improve renal outcomes in patients with HFrEF, supporting the role of dapagliflozin and empagliflozin as a new standard of care for patients with HFrEF (119, 122).
Sotagliflozin, another SGLT-2 inhibitor that displays also gastrointestinal SGLT-1 inhibition and thus reduces intestinal glucose absorption, was investigated in patients with type 2 diabetes after a recent hospitalization for worsening heart failure (SOLOIST-WHF). Patients were included independent of their ejection fraction, and 78% of patients had an ejection fraction <50%. The primary endpoint of CV death, total hospitalizations, and urgent visits for HF was significantly reduced in patients treated with sotagliflozin (HR: 0.67; 95% CI: 0.52–0.85; P < 0.001). The results were consistent among subgroups and especially also in patients with an EF > 50% (123). Sotagliflozin was also investigated in patients with type 2 diabetes, chronic kidney disease, and elevated CV risk (SCORED) (124); primary endpoint (changed during the study to a composite of CV death, total HF hospitalizations and urgent visits for HF) was significantly reduced in patients treated with sotagliflozin (HR: 0.67; 95% CI: 0.52–0.85; P < 0.001). It has to be mentioned that both sotagliflozin trials had to be stopped earlier than planned because of loss of funding from the sponsor.
Activators of soluble guanylate cyclase (victoria, vitality, capacity)
The activator of soluble guanylate cyclase (sGC) vericiguat was investigated in the VICTORIA study in 5050 patients with recently decompensated chronic HF and LVEF < 45% (125, 126). Vericiguat significantly reduced the primary outcome of CV death or first HF hospitalisation (HR: 0.90; 95% CI: 0.82–0.98; P = 0.02) (Figure 3). While vericiguat significantly reduced HF hospitalisations (HR: 0.90; 95% CI: 0.81–1.00), CV deaths were not significantly diminished. Adverse events were largely similar among the vericiguat and placebo groups. An analysis comparing HRs and absolute RR in three large recent HFrEF trials demonstrated that while the HR suggests a smaller treatment effect in VICTORIA than in the DAPA-HF and PARADIGM-HF trials, a comparison of 12-month event rates for the primary outcome pointed to a comparable benefit across the three trials (127, 128).
Given the significant interaction of vericiguat effects according to baseline NT-proBNP levels, a post hoc analysis showed an association of vericiguat benefit on the primary outcome in patients with NTproBNP levels up to 8000 pg/mL, with greatest benefit in patients with NTproBNP <4000 pg/mL (HR: 0.77, 95% CI: 0.68–0.88) (129). Vericiguat was evaluated In HFpEF patients in the VITALITY trial (128), showing no benefit in quality of life and exercise tolerance (130). Similarly, in the CAPACITY trial, the sGC stimulator praliciguat was well-tolerated but did neither affect the primary efficacy endpoint of pVO2 nor other predefined outcome parameters (131).
Cardiac myosin activators and inhibitors
Omecantiv-mecarbil (GALACTIC-HF, EXPLORER-HCM)
Omecamtiv-mecarbil, a cardiac myosin activator that enhances cardiomyocyte contraction, given twice daily on the basis of plasma levels of the drug, significantly reduced the primary endpoint of HF hospitalisation and CV death in patients with HFrEF and a recent HF event (HR: 0.92; 95% CI: 0.86–0.99; P = 0.03) (Figure 3) but had no impact on any of the secondary outcomes (CV death, change in symptom score, first HF hospitalization, and death from any cause) (132). A similar compound, danicamtiv, increased stroke volume, improved global longitudinal and circumferential strain, decreased LA minimal volume index, and increased LA function index when compared to placebo in a small phase 2a trial in 40 patients with stable HFrEF (133). On the other hand, mavacamten, a myosin inhibitor, significantly improved the combined primary endpoint of increase in peak oxygen consumption (pVO2) and reduction in NYHA class in a phase 3 trial in patients with obstructive hypertrophic cardiomyopathy. Also, outflow tract obstruction and health status were improved (134).
Other therapies
Ferric carboxymaltose (AFFIRM-AHF)
In iron-deficient patients hospitalized for acute HF (AFFIRM-AHF) (135), intravenous ferric carboxymaltose compared to placebo was associated with a trend to reduced total HF hospitalizations and CV death (rate ratio 0.79, 95% CI: 0.62–1.01, P = 0.059). In a pre-specified sensitivity analysis considering the impact of the COVID-19 pandemic, a statistically significant difference in favour of ferric carboxymaltose was reported for the primary endpoint was reported, but not in CV death risk (136).
MicroRNA-132 inhibition
In a first clinical trial limited by a small number of HF patients, the antisense oligonucleotide drug directed against miR-132, CDR132L (137), was well tolerated and showed first hints for a cardiac functional improvement (138).
Comprehensive disease-modifying pharmacological therapies
Using data from the EMPHASIS-HF, PARADIGM-HF, and DAPA-HF trials lifetime gains in survival have been estimated with comprehensive therapy (SV, β-blocker, MRA, and SGLT-2 inhibitor) vs. RAAS and β-blockers in patients with chronic HFrEF (11, 139). The HR for the composite endpoint of CV death or hospitalisation for HF was 0.38 (95% CI: 0.30–0.47). Favourable results were also calculated for CV death alone, hospitalization for HF alone, and all-cause mortality.
Comprehensive therapy could prolong overall survival 6.3 years in average in a 55-year-old patient. These results support the combination use of SV, β-blockers, mineralocorticoid receptor antagonists, and SGLT-2 inhibitors as a new therapeutic standard.
Device/interventional therapies
Secondary (or functional) mitral regurgitation (COAPT)
Secondary (or functional) mitral regurgitation (SMR) occurs frequently in HFrEF and is associated with progressive symptoms and worse prognosis. If SMR is treated by edge-to-edge repair, patients with optimal result at discharge and 12-month follow-up displayed best outcomes (140).
Cardiac resynchronization therapy (STOP-CRT)
Cardiac resynchronization therapy (STOP-CRT) is an integral part of treatment in patients with HFrEF, especially with left bundle branch block and wide QRS. In a selected cohort of patients with LVEF >50% during CRT and neurohormonal blockade, the STOP-CRT study investigated the feasibility and safety of neurohormonal blocker withdrawal. The incidence of adverse LV remodeling or clinical outcomes was low after discontinuation of beta-blockade/RAAS inhibition. However, comorbidities prompted the continuation of neurohormonal blockers in many patients (141).
In patients with HFrEF who are ineligible for CRT, baroreflex activation therapy (BAT) may be useful in addition to optimal drug therapy. In the BeAT-HF study, BAT was safe and significantly improved symptoms, quality of life, exercise capacity, and NT-proBNP (142). On the basis of these data, BAT was approved in the USA, while ongoing follow-up in the BeAT-HF study will assess effects on hard outcomes.
Specific management issues
Telemedicine and remote monitoring
The role of telemedicine and remote monitoring in the management of HF patients is still controversial. An observational study in three European countries showed that pulmonary artery pressure-guided HF management is feasible and safe and associated with better outcomes haemodynamic and clinical outcomes (143). Also, preliminary results testing non-invasive remote physiological monitoring from a wearable sensor showed promising results in the early detection of impending HF rehospitalisation (144).
However, different modes of remote monitoring failed to show a benefit in improving treatment, quality of life (145) or clinical outcomes (146). Moreover, remote monitoring with a cardiac implanted electronic device increased clinical activity for patients with HF and AF, with no associated reduction in mortality, and conversely, greater risk of CV hospitalisation amongst patients with persistent/permanent AF (147). In the COVID-19 era, remote monitoring is a useful tool for managing HF patients (148).
Self-care and palliative care
Self-care is essential in the management of chronic HF. Practical advice for key activities and priorities for self-care is given in an HFA manuscript (149). At the end of the HF pathway, palliative care should be introduced early, focusing on symptom management (150) regardless of prognosis, but actually only a minority in Europe receive it (151). Providing palliative care substantially reduces hospitalizations, with no clear adverse effect on survival (152).
Funding
There was no specific funding for the development of this manuscript. J. Bauersachs is supported by the Deutsche Forschungsgemeinschaft, KFO 311, “Advanced cardiac and pulmonary failure: mechanical unloading and repair”.
Disclosures: Dr. Bueno reports grants from Instituto de Salud Carlos III, grants from Sociedad Española de Cardiología, grants from Astra-Zeneca, grants and personal fees from Bayer, grants and personal fees from BMS, grants and personal fees from Novartis.
Dr. Moura reports personal fees from Astra Zeneca, personal fees from Vifor, personal fees from Servier, personal fees from Novartis, personal fees from Merck Serono, personal fees from Elly -Lilly, personal fees from Boerhringer-Ingelheim.
Dr. Bauersachs reports personal fees from Abbott, grants and personal fees from Abiomed, personal fees from Astra Zeneca, personal fees from Bayer, personal fees from BMS, personal fees from Boehringer Ingelheim, grants and personal fees from CvRX, personal fees from Daiichi Sankyo, personal fees from Medtronic, personal fees from MSD, personal fees from Novartis, personal fees from Pfizer, personal fees from Servier, grants and personal fees from Vifor, grants from Zoll, personal fees from Cardior. In addition, Dr. Bauersachs is Board Member of Cardior and has a patent PCT/EP2007/008772 with royalties paid, and a patent PCT/EP2009/051986 with royalties paid both on microRNA (miRNA) and downstream targets for diagnostic and therapeutic.
Dr. Lancellotti has no relevant disclosures.
References
1. Groenewegen A, Rutten FH, Mosterd A, et al. Epidemiology of heart failure. Eur J Heart Fail 2020; 22: 1342–1356.
2. Sulo G, Igland J, Øverland S, et al. Heart failure in Norway, 2000-2014: analysing incident, total and readmission rates using data from the Cardiovascular Disease in Norway (CVDNOR) Project. Eur J Heart Fail 2020; 22: 241–248.
3. Uijl A, Koudstaal S, Direk K, et al. Risk factors for incident heart failure in age- and sex-specific strata: a population-based cohort using linked electronic health records. Eur J Heart Fail 2019; 21: 1197–1206.
4. White-Williams C, Rossi LP, Bittner VA, et al. On behalf of the American Heart Association Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; and Council on Epidemiology and Prevention. Addressing social determinants of health in the care of patients with heart failure: a scientific statement from the American Heart Association. Circulation 2020 Jun; 141: e841–63.
5. Velagaleti RS, Larson MG, Enserro D, et al. Clinical course after a first episode of heart failure: insights from the Framingham Heart Study. Eur J Heart Fail 2020; 22: 1768–1776.
6. Tayal B, Fruelund P, Sogaard P, et al. Incidence of heart failure after pacemaker implantation: a nationwide Danish Registry-based follow-up study. Eur Heart J 2019; 40: 3641–3648.
7. Barra S, Providencia R, Narayanan K, et al. Time trends in sudden cardiac death risk in heart failure patients with cardiac resynchronization therapy: a systematic review. Eur Heart J 2020; 41: 1976–1986.
8. Motiejunaite J, Akiyama E, Cohen-Solal A, et al. The association of long-term outcome and biological sex in patients with acute heart failure from different geographic regions. Eur Heart J 2020; 41: 1357–1364.
9. Parizo JT, Kohsaka S, Sandhu AT, et al. Trends in readmission and mortality rates following heart failure hospitalization in the Veterans Affairs Health Care System from 2007 to 2017. JAMA Cardiol 2020; 5: 1042–1047.
10. Butt JH, Fosbøl EL, Gerds TA, et al. Readmission and death in patients admitted with new-onset versus worsening of chronic heart failure: insights from a nationwide cohort. Eur J Heart Fail 2020; 22: 1777–1785.
11. Jhund PS. The recurring problem of heart failure hospitalisations. Eur J Heart Fail 2020; 22: 249–250.
12. Celutkiene J, Lainscak M, Anderson L, et al. Imaging in patients with suspected acute heart failure: timeline approach position statement on behalf of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2020; 22: 181–195.
13. Paterson DI, Wells G, Erthal F, et al. OUTSMART HF: a randomized controlled trial of routine versus selective cardiac magnetic resonance for patients with non-ischemic heart failure (IMAGE-HF 1B). Circulation 2020; 141: 818–827.
14. Quarta G, Gori M, Iorio A, et al. Cardiac magnetic resonance in heart failure with preserved ejection fraction: myocyte, interstitium, microvascular, and metabolic abnormalities. Eur J Heart Fail 2020; 22: 1065–1075.
15. Pezel T, Viallon M, Croisille P, et al. Imaging interstitial fibrosis, left ventricular remodeling, and function in stage A and B heart failure. JACC Cardiovasc Imaging 2020. 10.1016/j.jcmg.2020.05.036 (accessed 24 December 2020).
16. Emrich T, Hahn F, Fleischmann D, et al. T1 and T2 mapping to detect chronic inflammation in cardiac magnetic resonance imaging in heart failure with reduced ejection fraction. ESC Hear Fail 2020; 7: 2544–2552.
17. Chamsi-Pasha MA, Zhan Y, Debs D, et al. CMR in the evaluation of diastolic dysfunction and phenotyping of HFpEF: current role and future perspectives. JACC Cardiovasc Imaging 2020; 13: 283–296.
18. Putko BN, Savu A, Kaul P, et al. Left atrial remodelling, mid-regional pro-atrial natriuretic peptide, and prognosis across a range of ejection fractions in heart failure. Eur Heart J Cardiovasc Imaging 2020. 10.1093/ehjci/jeaa041(accessed 24 December 2020).
19. Guazzi M, Ghio S, Adir Y. Pulmonary hypertension in HFpEF and HFrEF: JACC review topic of the week. J Am Coll Cardiol 2020; 76: 1102–1111.
20. Lin T-T, Wang Y-C, Juang J-MJ, et al. Application of the newest European Association of Cardiovascular Imaging Recommendation regarding the long-term prognostic relevance of left ventricular diastolic function in heart failure with preserved ejection fraction. Eur Radiol 2020; 30: 630–639.
21. Romano G, Magro S, Agnese V, et al. Clemenza F, Bellavia D. Echocardiography to estimate high filling pressure in patients with heart failure and reduced ejection fraction. ESC Hear Fail 2020; 7: 2268–2277.
22. Tanacli R, Hashemi D, Neye M, et al. Multilayer myocardial strain improves the diagnosis of heart failure with preserved ejection fraction. ESC Hear Fail 2020; 7: 3240–3245.
23. Selvaraj S, Myhre PL, Vaduganathan M, et al. Application of diagnostic algorithms for heart failure with preserved ejection fraction to the community. JACC Heart Fail 2020; 8: 640–653.
24. Yoshihisa A, Sato Y, Kanno Y, et al. Prognostic impacts of changes in left ventricular ejection fraction in heart failure patients with preserved left ventricular ejection fraction. Open Heart 2020; 7: e001112.
25. Wang C-L, Chan Y-H, Wu VC-C, et al. Incremental prognostic value of global myocardial work over ejection fraction and global longitudinal strain in patients with heart failure and reduced ejection fraction. Eur Heart J Cardiovasc Imaging 2020.
https://doi.org/10.1093/ehjci/jeaa162.
26. Mazzetti S, Scifo C, Abete R, et al. Short-term echocardiographic evaluation by global longitudinal strain in patients with heart failure treated with sacubitril/valsartan. ESC Hear Fail 2020; 7: 964–972.
27. Zhou W, Brown JM, Bajaj NS, et al. Hypertensive coronary microvascular dysfunction: a subclinical marker of end organ damage and heart failure. Eur Heart J 2020; 41: 2366–2375.
28. Escaned J, Lerman LO. Coronary microcirculation and hypertensive heart failure. Eur Heart J 2020; 41: 2376–2378.
29. Seo M, Yamada T, Tamaki S, et al. Prognostic significance of cardiac I-123-metaiodobenzylguanidine imaging in patients with reduced, mid-range, and preserved left ventricular ejection fraction admitted for acute decompensated heart failure: a prospective study in Osaka Prefectural Acute. Eur Heart J Cardiovasc Imaging 2020. https: //doi: 10.1093/ehjci/jeaa025 (accessed 24 December 2020).
30. Cunningham JW, Claggett BL, O’Meara E, et al. Effect of sacubitril/valsartan on biomarkers of extracellular matrix regulation in patients with HFpEF. J Am Coll Cardiol 2020; 76: 503–514.
31. Aimo A, Januzzi JL, Vergaro G, et al. Circulating levels and prognostic value of soluble ST2 in heart failure are less influenced by age than N-terminal pro-B-type natriuretic peptide and high-sensitivity troponin T. Eur J Heart Fail 2020; 22: 2078–2088.
32. Ferreira JP, Ouwerkerk W, Santema BT, et al. Differences in biomarkers and molecular pathways according to age for patients with HFrEF. Cardiovasc Res 2020. hhtps: //doi.org/10.1093/cvr/cvaa279.
33. Möckel M, Boer RA, Slagman AC, et al. Improve management of acute heart failure with ProcAlCiTonin in EUrope: results of the randomized clinical trial IMPACT EU Biomarkers in Cardiology (BIC) 18. Eur J Heart Fail 2020; 22: 267–275.
34. Bayes-Genis A, Liu PP, Lanfear DE, et al. Omics phenotyping in heart failure: the next frontier. Eur Heart J 2020; 41: 3477–3484.
35. McGranaghan P, Düngen H-D, Saxena A, et al. Incremental prognostic value of a novel metabolite-based biomarker score in congestive heart failure patients. ESC Hear Fail 2020; 7: 3029–3039.
36. Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020; 181: 271–280.e8.
37. Inciardi RM, Adamo M, Lupi L, et al. Characteristics and outcomes of patients hospitalized for COVID-19 and cardiac disease in Northern Italy. Eur Heart J 2020; 41: 1821–1829.
38. Sama IE, Ravera A, Santema BT, et al. Circulating plasma concentrations of angiotensin-converting enzyme 2 in men and women with heart failure and effects of renin-angiotensin-aldosterone inhibitors. Eur Heart J 2020; 41: 1810–1817.
39. Tomasoni D, Italia L, Adamo M, et al. COVID-19 and heart failure: from infection to inflammation and angiotensin II stimulation. Searching for evidence from a new disease. Eur J Heart Fail 2020; 22: 957–966.
40. de Abajo FJ, Rodríguez-Martín S, Lerma V, et al. Use of renin-angiotensin-aldosterone system inhibitors and risk of COVID-19 requiring admission to hospital: a case-population study. Lancet 2020; 395: 1705–1714.
41. Bean DM, Kraljevic Z, Searle T, Bendayan R, et al. Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers are not associated with severe COVID-19 infection in a multi-site UK acute hospital trust. Eur J Heart Fail 2020; 22: 967–974.
42. Reynolds HR, Adhikari S, Pulgarin C, et al. Renin-angiotensin-aldosterone system inhibitors and risk of Covid-19. N Engl J Med 2020; 382: 2441–2448.
43. Mancia G, Rea F, Ludergnani M, et al. Renin-angiotensin-aldosterone system blockers and the risk of Covid-19. N Engl J Med 2020; 382: 2431–2440.
44. Rey JR, Caro-Codón J, Rosillo SO, et al. Heart failure in Covid-19 patients: prevalence, incidence and prognostic implications. Eur J Heart Fail 2020. 10.1002/ejhf.1990.
45. Bromage DI, Cannatá A, Rind IA, et al. The impact of COVID-19 on heart failure hospitalization and management: report from a Heart Failure Unit in London during the peak of the pandemic. Eur J Heart Fail 2020; 22: 978–984.
46. Andersson C, Gerds T, Fosbøl E, et al. Incidence of new-onset and worsening heart failure before and after the COVID-19 epidemic lockdown in Denmark: a nationwide cohort study. Circ Heart Fail 2020; 13: e007274.
47. Cannata A, Bromage DI, Rind IA, et al. Temporal trends in decompensated heart failure and outcomes during COVID-19: a multisite report from heart failure referral centres in London. Eur J Heart Fail 2020. 10.1002/ejhf.1986 (accessed 24 December 2020).
48. Zhang Y, Coats AJS, Zheng Z, et al. Management of heart failure patients with COVID-19: a joint position paper of the Chinese Heart Failure Association & National Heart Failure Committee and the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2020; 22: 941–956.
49. D’Amario D, Restivo A, Canonico F, et al. Experience of remote cardiac care during the COVID-19 pandemic: the V-LAPTM device in advanced heart failure. Eur J Heart Fail 2020; 22: 1050–1052.
50. Anker SD, Butler J, Khan MS, et al. Conducting clinical trials in heart failure during (and after) the COVID-19 pandemic: an Expert Consensus Position Paper from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J 2020; 41: 2109–2117.
51. Truby LK, O’Connor C, Fiuzat M, et al. Sex differences in quality of life and clinical outcomes in patients with advanced heart failure: insights from the PAL-HF trial. Circ Heart Fail 2020; 13: e006134.
52. Lainscak M, Milinkovic I, Polovina M, et al. on behalf of the European Society of Cardiology Heart Failure Long-Term Registry Investigators Group. Sex- and age-related differences in the management and outcomes of chronic heart failure: an analysis of patients from the ESC HFA EORP Heart Failure Long-Term Registry. Eur J Heart Fail 2020; 22: 92–102.
53. Rossello X, Ferreira JP, Pocock SJ, et al. Sex differences in mineralocorticoid receptor antagonist trials: a pooled analysis of three large clinical trials. Eur J Heart Fail 2020; 22: 834–844.
54. Dewan P, Jackson A, Lam CSP, et al. Interactions between left ventricular ejection fraction, sex and effect of neurohumoral modulators in heart failure. Eur J Heart Fail 2020; 22: 898–901.
55. Reza N, Tahhan AS, Mahmud N, et al. Representation of women authors in international heart failure guidelines and contemporary clinical trials. Circ Heart Fail 2020; 13: e006605.
56. Pandey A, Vaduganathan M, Arora S, et al. Temporal trends in prevalence and prognostic implications of comorbidities among patients with acute decompensated heart failure: the ARIC study community surveillance. Circulation 2020; 142: 230–243.
57. Khan MS, Samman Tahhan A, Vaduganathan M, et al. Trends in prevalence of comorbidities in heart failure clinical trials. Eur J Heart Fail 2020; 22: 1032–1042.
58. Bhatt AS, Ambrosy AP, Dunning A, et al. The burden of non-cardiac comorbidities and association with clinical outcomes in an acute heart failure trial – insights from ASCEND-HF. Eur J Heart Fail 2020; 22: 1022–1031.
59. Aimo A, Barison A, Castiglione V, et al. The unbearable underreporting of comorbidities in heart failure clinical trials. Eur J Heart Fail 2020; 22: 1043–1044.
60. Docherty KF, Shen L, Castagno D, et al. Relationship between heart rate and outcomes in patients in sinus rhythm or atrial fibrillation with heart failure and reduced ejection fraction. Eur J Heart Fail 2020; 22: 528–538.
61. Al-Khatib SM, Benjamin EJ, Albert CM, et al. Advancing research on the complex interrelations between atrial fibrillation and heart failure: a report from a US National Heart, Lung, and Blood Institute Virtual Workshop. Circulation 2020; 141: 1915–1926.
62. Packer M. Do most patients with obesity or type 2 diabetes, and atrial fibrillation, also have undiagnosed heart failure? A critical conceptual framework for understanding mechanisms and improving diagnosis and treatment. Eur J Heart Fail 2020; 22: 214–227.
63. Bauersachs J, Veltmann C. Heart rate control in heart failure with reduced ejection fraction: the bright and the dark side of the moon. Eur J Heart Fail 2020; 22: 539–542.
64. Mullens W, Damman K, Testani JM, et al. Evaluation of kidney function throughout the heart failure trajectory – a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2020; 22: 584–603.
65. Cox ZL, Hung R, Lenihan DJ, et al. Diuretic strategies for loop diuretic resistance in acute heart failure: the 3T trial. JACC Heart Fail 2020; 8: 157–168.
66. Carubelli V, Zhang Y, Metra M, et al. the Istaroxime ADHF Trial Group. Treatment with 24 hour istaroxime infusion in patients hospitalized for acute heart failure: a randomised, placebo-controlled trial. Eur J Heart Fail 2020; 22: 1684–1693.
67. Harjola V-P, Parissis J, Bauersachs J, et al. Acute coronary syndromes and acute heart failure: a diagnostic dilemma and high-risk combination. A statement from the Acute Heart Failure Committee of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2020; 22: 1298–1314.
68. Gorenek B, Halvorsen S, Kudaiberdieva G, et al. Atrial fibrillation in acute heart failure: a position statement from the Acute Cardiovascular Care Association and European Heart Rhythm Association of the European Society of Cardiology. Eur Hear J Acute Cardiovasc Care 2020; 9: 348–357.
69. Aissaoui N, Puymirat E, Delmas C, et al. Trends in cardiogenic shock complicating acute myocardial infarction. Eur J Heart Fail 2020; 22: 664–672.
70. Hanson ID, Tagami T, Mando R, et al. National Cardiogenic Shock Investigators. SCAI shock classification in acute myocardial infarction: insights from the National Cardiogenic Shock Initiative. Catheter Cardiovasc Interv Off J Soc Card Angiogr Interv 2020; 96: 1137–1142.
71. Zeymer U, Bueno H, Granger CB, et al. Acute Cardiovascular Care Association position statement for the diagnosis and treatment of patients with acute myocardial infarction complicated by cardiogenic shock: a document of the Acute Cardiovascular Care Association of the European Society of Car. Eur Hear J Acute Cardiovasc Care 2020; 9: 183–197.
72. Chioncel O, Parissis J, Mebazaa A, et al. Epidemiology, pathophysiology and contemporary management of cardiogenic shock—a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2020; 22: 1315–1341.
73. Karason K, Lund LH, Dalén M, et al. the SweVAD Investigators. Randomized trial of a left ventricular assist device as destination therapy versus guideline-directed medical therapy in patients with advanced heart failure. Rationale and design of the SWEdish evaluation of left Ventricular Assist Device (SweVAD) trial. Eur J Heart Fail 2020; 22: 739–750.
74. Phan D, Duan L, Ng A, et al. Characteristics and outcomes of pregnant women with cardiomyopathy stratified by etiologies: a population based study. Int J Cardiol 2020; 305: 87–91.
75. Bauersachs J, König T, Meer P, et al. Pathophysiology, diagnosis and management of peripartum cardiomyopathy: a position statement from the Heart Failure Association of the European Society of Cardiology Study Group on peripartum cardiomyopathy. Eur J Heart Fail 2019; 21: 827–843.
76. Sliwa K, Bauersachs J, Coats AJS. The European Society of Cardiology Heart Failure Association Study Group on Peripartum Cardiomyopathy – what has been achieved in 10 years. Eur J Heart Fail 2020; 22: 1060–1064.
77. Sliwa K, Petrie MC, van der Meer P, et al. Clinical presentation, management, and 6-month outcomes in women with peripartum cardiomyopathy: an ESC EORP registry. Eur Heart J 2020; 41: 3787–3797.
78. Davis MB, Arany Z, McNamara DM, et al. Peripartum cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol 2020; 75: 207–221.
79. Wilcox JE, Fang JC, Margulies KB, et al. Heart Failure With recovered left ventricular ejection fraction: JACC Scientific Expert Panel. J Am Coll Cardiol 2020; 76: 719–734.
80. Curigliano G, Lenihan D, Fradley M, et al. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann Oncol Off J Eur Soc Med Oncol 2020; 31: 171–190.
81. Keramida K, Farmakis D, López Fernández T, et al. Focused echocardiography in cardio-oncology. Echocardiography 2020; 37: 1149–1158.
82. Harries I, Liang K, Williams M, et al. Magnetic resonance imaging to detect cardiovascular effects of cancer therapy. JACC CardioOncology 2020; 2: 270–292.
83. Zhang L, Awadalla M, Mahmood SS, et al. Cardiovascular magnetic resonance in immune checkpoint inhibitorassociated myocarditis. Eur Heart J 2020; 41: 1733–1743.
84. Border WL, Sachdeva R, Stratton KL, et al. Longitudinal changes in echocardiographic parameters of cardiac function in pediatric cancer survivors. JACC CardioOncology 2020; 2: 26–37.
85. López-Sendón J, A´ lvarez-Ortega C, Zamora Aunon P, et al. Classification, prevalence, and outcomes of anticancer therapy-induced cardiotoxicity: the CARDIOTOX registry. Eur Heart J 2020; 41: 1720–1729.
86. Reddy YNV, Obokata M, Wiley B, et al. The haemodynamic basis of lung congestion during exercise in heart failure with preserved ejection fraction. Eur Heart J 2019; 40: 3721–3730.
87. Obokata M, Kane GC, Reddy YNV, et al. The neurohormonal basis of pulmonary hypertension in heart failure with preserved ejection fraction. Eur Heart J 2019; 40: 3707–3717.
88. Padang R, Chandrashekar N, Indrabhinduwat M, et al. Aetiology and outcomes of severe right ventricular dysfunction. Eur Heart J 2020; 41: 1273–1282.
89. Patel D, Trulock K, Kumar A, et al. Baseline right ventricular dysfunction predicts worse outcomes in patients undergoing cardiac resynchronization therapy implantation. J Card Fail 2020; 26: 227–232.
90. Rohde LE, Chatterjee NA, Vaduganathan M, et al. Sacubitril/valsartan and sudden cardiac death according to implantable cardioverter-defibrillator use and heart failure cause: a PARADIGM-HF analysis. JACC Heart Fail 2020; 8: 844–855.
91. Lee S, Oh J, Kim H, et al. Sacubitril/valsartan in patients with heart failure with reduced ejection fraction with end-stage of renal disease. ESC Hear Fail 2020; 7: 1125–1129.
92. Mann DL, Greene SJ, Givertz MM, et al. Sacubitril/valsartan in advanced heart failure with reduced ejection fraction: rationale and design of the lIFE trial. JACC Heart Fail 2020; 8: 789–999.
93. McMurray JJV, Jackson AM, Lam CSP, et al. Effects of sacubitril-valsartan versus valsartan in women compared with men with heart failure and preserved ejection fraction: insights from PARAGON-HF. Circulation 2020; 141: 338–351
94. Vaduganathan M, Claggett BL, Desai AS, et al. Prior heart failure hospitalization, clinical outcomes, and response to sacubitril/valsartan compared with valsartan in HFpEF. J Am Coll Cardiol 2020; 75: 245–254.
95. Selvaraj S, Claggett BL, Böhm M, et al. Systolic blood pressure in heart failure with preserved ejection fraction treated with sacubitril/valsartan. J Am Coll Cardiol 2020; 75: 1644–1656.
96. Mc Causland FR, Lefkowitz MP, Claggett B, et al. Angiotensin-neprilysin inhibition and renal outcomes in heart failure with preserved ejection fraction. Circulation 2020; 142: 1236–1245.
97. Selvaraj S, Claggett BL, Pfeffer MA, et al. Serum uric acid, influence of sacubitril/valsartan, and cardiovascular outcomes in heart failure with preserved ejection fraction: PARAGON-HF. Eur J Heart Fail 2020; 22: 2093–2101.
98. Kuno T, Ueyama H, Fujisaki T, et al. Meta-analysis evaluating the effects of renin-angiotensin-aldosterone system blockade on outcomes of heart failure with preserved ejection fraction. Am J Cardiol 2020; 125: 1187–1193.
99. Vaduganathan M, Jhund PS, Claggett BL, et al. A putative placebo analysis of the effects of sacubitril/valsartan in heart failure across the full range of ejection fraction. Eur Heart J 2020; 41: 2356–2362.
100. Böhm M, Bewarder Y, Kindermann I. Ejection fraction in heart failure revisited— where does the evidence start? Eur Heart J 2020; 41: 2363–2365.
101. Seferovic PM, Fragasso G, Petrie M, et al. Sodium-glucose cotransporter 2 inhibitors in heart failure: beyond glycaemic control. The position paper of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2020; 22: 1495–1503.
102. Seferovic PM, Coats AJS, Ponikowski P, et al. European Society of Cardiology/Heart Failure Association position paper on the role and safety of new glucose-lowering drugs in patients with heart failure. Eur J Heart Fail 2020; 22: 196–213.
103. Cannon CP, Pratley R, Dagogo-Jack S, et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med 2020; 383: 1425–1435.
104. Cosentino F, Cannon CP, Cherney DZI, et al. On behalf of the VERTIS CV Investigators. Efficacy of ertugliflozin on heart failure-related events in patients with type 2 diabetes mellitus and established atherosclerotic cardiovascular disease: results of the VERTIS CV trial. Circulation 2020; 142: 2205–2215.
105. McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019; 381: 1995–2008.
106. McMurray JJV, DeMets DL, Inzucchi SE, et al. on behalf of the DAPA-HF Committees and Investigators. A trial to evaluate the effect of the sodium-glucose co-transporter 2 inhibitor dapagliflozin on morbidity and mortality in patients with heart failure and reduced left ventricular ejection fraction (DAPA-HF). Eur J Heart Fail 2019; 21: 665–675.
107. Petrie MC, Verma S, Docherty KF, et al. Effect of dapagliflozin on worsening heart failure and cardiovascular death in patients with heart failure with and without diabetes. JAMA 2020; 323: 1353–1368.
108. Docherty KF, Jhund PS, Inzucchi SE, et al. Effects of dapagliflozin in DAPA-HF according to background heart failure therapy. Eur Heart J 2020; 41: 2379–2392.
109. Martinez FA, Serenelli M, Nicolau JC, et al. Efficacy and safety of dapagliflozin in heart failure with reduced ejection fraction according to age: insights from DAPA-HF. Circulation 2020; 141: 100–111.
110. Serenelli M, Böhm M, Inzucchi SE, et al. Effect of dapagliflozin according to baseline systolic blood pressure in the Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure trial (DAPA-HF). Eur Heart J 2020; 41: 3402–3418.
111. Jhund PS, Solomon SD, Docherty KF, et al. Efficacy of dapagliflozin on renal function and outcomes in patients with heart failure with reduced ejection fraction: results of DAPA-HF. Circulation 2020. 10.1161/CIRCULATIONAHA.120.050391 (accessed 24 December 2020).
112. Kosiborod MN, Jhund PS, Docherty KF, et al. Effects of dapagliflozin on symptoms, function, and quality of life in patients with heart failure and reduced ejection fraction: results from the DAPA-HF trial. Circulation 2020; 141: 90–99.
113. McEwan P, Darlington O, McMurray JJV, et al. Cost-effectiveness of dapagliflozin as a treatment for heart failure with reduced ejection fraction: a multinational health economic analysis of DAPA-HF. Eur J Heart Fail 2020; 22: 2147–2156.
114. Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020; 383: 1436–1446.
115. Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020; 383: 1413–1424.
116. Packer M, Anker SD, Butler J, et al. Effect of empagliflozin on the clinical stability of patients with heart failure and a reduced ejection fraction: the EMPEROR-Reduced trial. Circulation 2020. 10.1161/CIRCULATIONAHA.120.051783 (accessed 24 December 2020).
117. Packer M. Influence of neprilysin inhibition on the efficacy and safety of empagliflozin in patients with chronic heart failure and a reduced ejection fraction: the EMPEROR-Reduced trial. Eur Hear J 2020, in press.
118. Anker SD, Butler J, Filippatos G, et al. Effect of empagliflozin on cardiovascular and renal outcomes in patients with heart failure by baseline diabetes status—results from the EMPEROR-Reduced trial. Circulation 2020. hhtps: //doi.org/10.1161/CIRCULATIONAHA.120.051824.
119. Zannad F, Ferreira JP, Pocock SJ, et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPERORReduced and DAPA-HF trials. Lancet (London, England) 2020; 396: 819–829.
120. Lee MMY, Brooksbank KJM, Wetherall K, et al. Effect of empagliflozin on left ventricular volumes in patients with type 2 diabetes, or prediabetes, and heart failure with reduced ejection fraction (SUGAR-DM-HF). Circulation 2020.
https://doi.org/10.1161/CIRCULATIONAHA.120.052186 (accessed 24 December 2020).
121. Santos-Gallego CG, Vargas-Delgado AP, Requena JA, et al. Randomized trial of empagliflozin in non-diabetic patients with heart failure and reduced ejection fraction. J Am Coll Cardiol 2020. 10.1016/j.jacc.2020.11.008.
122. Butler J, Zannad F, Filippatos G, et al. Totality of evidence in trials of sodium-glucose co-transporter-2 inhibitors in the patients with heart failure with reduced ejection fraction: implications for clinical practice. Eur Heart J 2020; 41: 3398–3401.
123. Bhatt DL, Szarek M, Steg PG, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med 2020. https://doi.org/10.1056/NEJMoa2030183 (accessed 24 December 2020).
124. Bhatt DL, Szarek M, Pitt B, Cannon CP, et al. Sotagliflozin in Patients with Diabetes and Chronic Kidney Disease. N Engl J Med 2020. https://doi.org/10.1056/NEJMoa2030186 (accessed 24 December 2020).
125. Pieske B, Patel MJ, Westerhout CM, et al. on behalf of the VICTORIA Study Group. Baseline features of the VICTORIA (Vericiguat Global Study in Subjects with Heart Failure with Reduced Ejection Fraction) trial. Eur J Heart Fail 2019; 21: 1596–1604.
126. Armstrong PW, Pieske B, Anstrom KJ, et al. Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med 2020; 382: 1883–1893.
127. Butler J, Anstrom KJ, Armstrong PW; For the VICTORIA Study Group. Comparing the benefit of novel therapies across clinical trials: insights from the VICTORIA trial. Circulation 2020; 142: 717–719.
128. Butler J, Lam CSP, Anstrom KJ, et al. Rationale and design of the VITALITY-HFpEF trial. Circ Heart Fail 2019; 12: e005998.
129. Ezekowitz JA, O’Connor CM, Troughton RW, et al. N-terminal Pro-B-type natriuretic peptide and clinical outcomes: vericiguat heart failure with reduced ejection fraction study. JACC Heart Fail 2020; 8: 931–939.
130. Armstrong PW, Lam CSP, Anstrom KJ, et al. VITALITY-HfpEF Study Group. Effect of vericiguat vs placebo on quality of life in patients with heart failure and preserved ejection fraction: the VITALITY-HFpEF randomized clinical trial. JAMA 2020; 324: 1512–1521. Oct
131. Udelson JE, Lewis GD, Shah SJ, et al. Rationale and design for a multicenter, randomized, double-blind, placebo-controlled, phase 2 study evaluating the safety and efficacy of the soluble guanylate cyclase stimulator praliciguat over 12 weeks in patients with heart failure with preserved eje. Am Heart J 2020; 222: 183–190.
132. Teerlink JR, Diaz R, Felker GM, et al. Cardiac myosin activation with omecamtiv mecarbil in systolic heart failure. N Engl J Med 2020. 10.1056/NEJMoa2025797 (accessed 24 December 2020).
133. Voors AA, Tamby J-F, Cleland JG, et al. Effects of danicamtiv, a novel cardiac myosin activator, in heart failure with reduced ejection fraction: experimental data and clinical results from a phase 2a trial. Eur J Heart Fail 2020; 22: 1649–1658.
134. Olivotto I, Oreziak A, Barriales-Villa R, et al. Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER-HCM): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2020; 396: 759–769.
135. Ponikowski P, Kirwan B-A, Anker SD, et al. Rationale and design of the AFFIRM-AHF trial: a randomised, double-blind, placebo-controlled trial comparing the effect of intravenous ferric carboxymaltose on hospitalisations and mortality in iron-deficient patients admitted for acute heart failure. Eur J Heart Fail 2019; 21: 1651–1658.
136. Ponikowski P, Kirwan B-A, Anker SD, et al. Ferric carboxymaltose for iron deficiency at discharge after acute heart failure: a multicentre, double-blind, randomised, controlled trial. Lancet (London, England) 2020. 10.1016/S0140-6736(20)32339-4 (accessed 24 December 2020).
137. Batkai S, Genschel C, Viereck J, et al. CDR132L improves systolic and diastolic function in a large animal model of chronic heart failure. Eur Heart J 2020. 10.1093/eurheartj/ehaa791 (accessed 24 December 2020).
138. Täubel J, Hauke W, Rump S, et al. Novel antisense therapy targeting microRNA-132 in patients with heart failure: results of a first-in-human Phase 1b randomized, double-blind, placebo-controlled study. Eur Heart J 2020. 10.1093/eurheartj/ehaa898 (accessed 24 December 2020).
139. Vaduganathan M, Claggett BL, Jhund PS, et al. Estimating lifetime benefits of comprehensive disease-modifying pharmacological therapies in patients with heart failure with reduced ejection fraction: a comparative analysis of three randomised controlled trials. Lancet (London, England) 2020; 396: 121–128.
140. Reichart D, Kalbacher D, Rübsamen N, Tigges E, Thomas C, Schirmer J, Reichenspurner H, Blankenberg S, Conradi L, Schäfer U, Lubos E. The impact of residual mitral regurgitation after MitraClip therapy in functional mitral regurgitation. Eur J Heart Fail 2020; 22: 1840–1848.
141. Nijst P, Martens P, Dauw J, et al. Withdrawal of neurohumoral blockade after cardiac resynchronization therapy. J Am Coll Cardiol 2020; 75: 1426–1438.
142. Zile MR, Lindenfeld JAnn, Weaver FA, et al. Baroreflex activation therapy in patients with heart failure with reduced ejection fraction. J Am Coll Cardiol 2020; 76: 1–13.
143. Angermann CE, Assmus B, Anker SD, et al. for the MEMS-HF Investigators. Pulmonary artery pressure-guided therapy in ambulatory patients with symptomatic heart failure: the CardioMEMS European Monitoring Study for Heart Failure (MEMS-HF). Eur J Heart Fail 2020; 22: 1891–1901. Jun
144. Stehlik J, Schmalfuss C, Bozkurt B, et al. Continuous wearable monitoring analytics predict heart failure hospitalization: the LINK-HF multicenter study. Circ Heart Fail 2020; 13: e006513.
145. Rahimi K, Nazarzadeh M, Pinho-Gomes A-C, et al. SUPPORT-HF2 Study Group. Home monitoring with technology-supported management in chronic heart failure: a randomised trial. Heart 2020; 106: 1573–1578. Oct
146. Galinier M, Roubille F, Berdague P, et al. on behalf of the OSICAT Investigators. Telemonitoring versus standard care in heart failure: a randomised multicentre trial. Eur J Heart Fail 2020; 22: 985–994.
147. Zakeri R, Morgan JM, Phillips P, et al. REM-HF Investigators. Impact of remote monitoring on clinical outcomes for patients with heart failure and atrial fibrillation: results from the REM-HF trial. Eur J Heart Fail 2020; 22: 543–553.
148. Abraham WT, Fiuzat M, Psotka MA, et al. Heart failure collaboratory statement on remote monitoring and social distancing in the landscape of COVID-19. JACC Heart Failure 2020; 8: 692–694.
149. Jaarsma T, Hill L, Bayes-Genis A, et al. Self-care of heart failure patients: practical management recommendations from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2020. 10.1002/ejhf.2008 (accessed 24 December 2020).
150. Hill L, Geller TP, Baruah R, et al. Integration of a palliative approach into heart failure care: a European Society of Cardiology Heart Failure Association position paper. Eur J Heart Fail 2020. 10.1002/ejhf.1994
151. Sobanski PZ, Alt-Epping B, Currow DC, et al. Palliative care for people living with heart failure: European Association for Palliative Care Task Force expert position statement. Cardiovasc Res 2020; 116: 12–27.
152. Sahlollbey N, Lee CKS, Shirin A, et al. The impact of palliative care on clinical and patient-centred outcomes in patients with advanced heart failure: a systematic review of randomized controlled trials. Eur J Heart Fail 2020. https: //doi.org/10.1002/ejhf.1783 (accessed 24 December 2020).