The year in cardiology 2017: prevention
█ Current opinion
Authors:
Børge G. Nordestgaard1*, Francesco Cosentino2, Ulf Landmesser3, and Ulrich Laufs4
1Department of Clinical Biochemistry and The Copenhagen General Population Study, Herlev and Gentofte Hospital, Copenhagen University Hospital, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark
2Cardiology Unit, Department of Medicine, Karolinska University Hospital, Solna, Stockholm, Sweden
3Department of Cardiology, Charite Universitätsmedizin Berlin, Berlin Institute of Health (BIH), German Center of Cardiovascular Research (DZHK), Hindenburgdamm 30, 12203 Berlin, Germany
4Klinik und Poliklinik für Kardiologie, Department für Innere Medizin, Neurologie und Dermatologie, Universitätsklinikum Leipzig, Leipzig, Germany
* Corresponding author:
Preamble
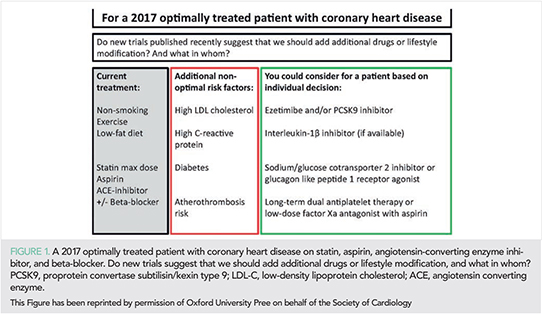
During 2017 several landmark studies have been published that have practical implications for atherosclerotic cardiovascular disease (ASCVD) prevention and risk factor control, such as lipids and lipoproteins, inflammation, diabetes, hypertension, and healthy lifestyle. We use the term “ASCVD” where relevant to simplify the reading of this article for the non-specialist, although the exact definition as ASCVD differ slightly from study to study. However, in sections where ASCVD clearly is not the relevant endpoint (e.g. in hypertension research) we do not use “ASCVD”, but instead of use other words to describe endpoints. All relevant trials have been performed on a background of optimal medical therapy, such as described in the European Society of Cardiology(ESC)/European Atherosclerosis Society(EAS) guidelines on ASCVD prevention and management of dyslipidaemia for lipid-lowering (1, 2). For example, important new evidence for additional risk reduction relates to lipid-lowering [proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibition (3), cholesteryl ester transfer protein (CETP) inhibition] (4) to the reduction of systemic inflammation (interleukin-1β inhibition) (5) and to anti-thrombotic therapy (low-dose factor Xa antagonism) (6). Since these novel treatments have not yet been tested in combination and because of the practical and economic limitations, an important challenge for the years to come is patient selection. Also, the benefit to risk dimension of any new therapeutic agent needs to be considered. This review article is intended to provide the practicing physician with the information needed to identify patients in secondary prevention that may benefit the most from additional novel treatments (Figure 1), and at the same time give a comprehensive update of novel insights relevant both to primary and secondary prevention of ASCVD. Use and accessibility of novel treatments will depend critically on whether patients live in high income, upper middle-income or lower middle-income countries, as levels of cardiovascular risk factors, cardiovascular mortality rates, and thus the prevention potential differ between such countries (7).
ISSUE: CARDIOLOGIA HUNGARICA | 2018 | VOLUME 48, ISSUE 6
Lifestyle
Observational epidemiology in the field of lifestyle is difficult to trust due to the high-risk of confounding (a third factor influences both disease risk and lifestyle) and reverse causation (diseases will change a person’s lifestyle), and therefore only randomized intervention trials and genetic Mendelian randomizations studies can be trusted. However, each of these study designs has limitations (8–10).
Importantly, as randomized intervention trials are very difficult to conduct for lifestyle factors, we often are left with observational and genetic studies in this field. Below is what we choose to highlight for 2017.
The concept of “metabolically healthy obesity”, namely that in the absence of metabolic dysfunction, individuals with excess adiposity are not at greater cardiovascular risk, has been controversial. A recent pan-European case-cohort study nested within the European Prospective Investigation into Cancer and Nutrition study (EPIC-CVD), observed higher cardiovascular risk with increasing general and central adiposity (11). Other cohort studies have challenged this concept reporting an excess of cardiovascular risk in metabolically healthy obese as compared to normal weight individuals (12–15). These results highlight the importance of population-wide prevention of obesity with lifestyle intervention targeting eating behaviour and physical activity. Importantly however, steady and sustained weight loss is preferable as in patients with coronary heart disease the highest vs. lowest variation in body weight was associated with 64% more coronary and 124% more mortality events (16).
Coffee consumption is observationally associated with reduced all-cause, cardiovascular and other cause-specific mortality (17–19). However, both reverse causation and confounding by other lifestyle factors may bias such results. Interestingly therefore, Mendelian randomization studies free of confounding found no causal effect of coffee intake on all-cause or cardiovascular mortality, or on cardiovascular disease (19). Likewise, in Mendelian randomization studies milk intake appears not to influence risk of hypertension or cardiovascular disease (20, 21).
Alcohol intake: novel findings include that acute beer alcohol consumption during the Munich Octoberfest was associated with cardiac arrhythmias and sinus tachycardia (22). Large UK and USA cohorts found moderate alcohol intake associated with less of most cardiovascular disease endpoints while heavy and binge drinking or alcohol abuse were associated with more cardiovascular disease or deaths (23–25).
A Mendelian randomization study of genotypes associated with higher education suggested that low education is causally associated with ASCVD events (26). Using UK-Biobank participants, it was observed that the association between physical activity and mortality was strongest in those with lowest strength and lowest cardiorespiratory fitness, suggesting that these subgroups would benefit the most from more physical activity (27); preventing or delaying cardiovascular disease or diabetes seemed to delay cognitive decline and possibly dementia (28).
Adherence to a healthy lifestyle consisting of non-smoking, light to moderate alcohol intake, high physical activity, fruit and vegetables intake, and normal body weight was associated with a substantially lower burden of ASCVD in Chinese (29, like previously observed in Europeans. Interestingly, in Spain skipping breakfast was associated with more non-coronary and generalized atherosclerosis, independent of other cardiovascular risk factors (30). Importantly however, lifestyle can be difficult to change, even for patients with acute coronary syndrome and/or revascularization (31).
Further, in the PURE study covering all major parts of the World and recruiting 135,335 individuals between 2003 and 2013 with follow-up until 2017, higher intake of fruit, vegetables, and legumes was associated with lower non-cardiovascular and total mortality, with a non-significant trend for cardiovascular mortality (32). The findings also included that as little as three servings per day consisting of only 375 g per day were associated with similar benefit. This indicate that optimal health benefits may be achieved with a more modest consumption of fruit, vegetables, and legumes than that recommended in high-income Europe and the USA, an approach that is more likely to be affordable in low-income and middle-income countries. In contrast to popular opinion, higher fat intake was not associated with ASCVD or death.
Finally, air pollution, noise, and other environmental stressors, depending on where a person chooses to live, may influence cardiovascular health and mortality (33, 34). For example, long-term exposure to road traffic noise and ambient air pollution were associated adversely with cardiovascular biochemical risk factors (35) and self-reported hypertension (36). Worldwide ambient air pollution with aerodynamic diameter <2.5 µm was the fifth-ranked mortality factor in 2015, and has increased in importance over 25 years (37).
Low-density lipoprotein cholesterol
One of the biggest potential for preventing ASCVD worldwide is to find and treat individuals with FH early in life. Because of the recognition that FH is found in roughly 1/250 (rather than 1/500) (54–56), because FH is underdiagnosed and undertreated (57–59), and because PCSK9 inhibitors together with statins now offer efficient LDL cholesterol reduction in FH, interest in FH research is increasing. After optimal statin therapy, PCSK9 inhibitors can reduce LDL cholesterol by an additional up to 65% in individuals with heterozygous FH, and up to the same absolute extent in the very rare individuals with homozygous FH but depending critically on the types of mutations involved and thus the ability to up-regulate LDL receptors.

A Japanese study documented that clinical signs of FH and FH mutations additively added to ASCVD risk above high LDL cholesterol alone (60); importantly however, except for the Netherlands, Norway, a number of other European countries, and Canada, in most countries in the World FH is underdiagnosed and genetic testing is not used (Take home figure) (61); although Japan has a relatively high rate of FH screening, genetic testing is still only used rarely in Japan. The advantage of genetic testing is the use in cascade screening of FH index cases and their family members (62), and such testing in the UK including consequent cholesterol-lowering treatment has an estimated lifetime cost per relative tested of only 1212 Euro (£1092) if 3.2 relatives are tested per mutation-positive index case (63). Thus, can we afford not to screen for FH (64)?

To better select individuals for genetic testing for FH, based on Dutch data with validation in Canada, an online calculator to estimate the probability of an FH mutation in individual patients has been developed (65). Further, among Spanish patients with acute coronary syndrome and LDL cholesterol ≥4.1 mmol/L (160 mg/dL), 9% had an FH mutation (66). Also, using the Dutch Lipid Clinic Network Criteria or simply a high LDL cholesterol alone also improved finding those with FH mutations (55); the most optimal threshold for LDL cholesterol concentration to discriminate between Danish mutation carriers and non-carriers was 4.4 mmol/L (170 mg/dL).
In children with homozygous FH rosuvastatin reduced LDL cholesterol by 22% (67) and in children with heterozygous FH rosuvastain slowed the progression of carotid intima-media thickness (68), supporting early statin therapy in children with FH. Interestingly, the pro-inflammatory phenotype of monocytes in FH patients was dampened by LDL cholesterol lowering (69). Further, based on the Spanish SAFEHEART registry, ASCVD risk prediction depended on age, sex, previous ASCVD, blood pressure, body mass index, smoking, LDL cholesterol, and lipoprotein(a) (70); an independent predictive value of lipoprotein(a) in FH for ASCVD agrees with previous findings (71). Finally, based on WOSCOPS trial 20-years follow-up data we can now say definitively that statin treatments of primary prevention patients with LDL-C ≥4.9 mmol/L (190 mg/dL) is safe and leads to significant reductions in ASCVD events and total mortality (72).
Lipoprotein(a)
Genetic evidence documents that lipoprotein(a) is causally related to myocardial infarction, atherosclerotic stenosis, and aortic valve stenosis, but not necessarily with development of early atherosclerosis (73, 74). Until now focus has been on genetic variants that increases lipoprotein(a) and increases disease risk, but in 2017 in individuals with small apolipoprotein(a) isoforms a novel genetic variant that reduces lipoprotein(a) and cardiovascular disease risk was documented (75). Another novel genetic approach include the use of genetic variants solely associated with lipoprotein(a) concentrations and not with number of kringle IV-2, or vice versa, to show that the higher diabetes risk observed at low lipoprotein(a) is explained by high kringle IV-2 and not by low lipoprotein(a) per se (76); for future aggressive lipoprotein(a) lowering (74, 77), this is a reassuring finding.
Across Europe lipoprotein(a) levels vary; however, high lipoprotein(a) was associated with high ASCVD risk in all regions (78); absolute lipoprotein(a) concentrations were lower in this study than in many others, pointing towards the need for further standardization of lipoprotein(a) measurements.
At present, statins are applied to individuals with high lipoprotein(a) to reduce ASCVD risk. Other investigational therapies include apheresis, PCSK9 inhibitors, and most importantly antisense oligonucleotides targeting apolipoprotein(a) production (74, 77).Interestingly and surprisingly, in a study of only 20 patients with refractory angina lipoprotein apheresis improved myocardial perfusion, atheroma burden, exercise capacity and symptoms (79); these findings may initiate studies in similar patients using PCSK9 inhibitors or antisense oligonucleotides (80).
Triglycerides and remnants
Three large randomized double-blind ASCVD endpoint trials of triglyceride-lowering with omega-3 fatty acids or pemafibrate in individuals already on a statin, the REDUCE-IT (NCT01492361), STRENGTH (NCT02104817), and PROMINENT (NCT03071692) trials, are now ongoing. In the meantime, the genetic evidence that triglyceride-rich lipoproteins and remnant cholesterol represent an independent cause of ASCVD beyond LDL cholesterol is increasing in strength (9); remnant cholesterol is the cholesterol content of all triglyceride-rich lipoproteins and can either be calculated (total minus LDL minus HDL cholesterol) or now also measured directly on standard hospital autoanalysers (81).
Individuals with loss-of-function mutations in angiopoietin-like protein 3 (ANGPTL3), a known inhibitor of triglyceride-degrading lipoprotein lipase, had 27% lower triglycerides, 9% lower LDL cholesterol, and 41% lower ASCVD risk (82); similar findings were observed in an independent study (83). Pharmacologically, antibodies against ANGPTL3 reduced triglycerides by up to 76% and LDL cholesterol up to 23%,82 while antisense oligonucleotides against ANGPTL3 messenger RNA reduced triglycerides by up to 63% and LDL cholesterol up to 33% (84).
Conversely, loss-of-function mutations in lipoprotein lipase lead to increased triglycerides and increased ASCVD risk (85), supporting earlier findings (86). Another novel observation include that autoantibodies against glycosylphosphatidylinositol-anchored HDL binding protein 1 (GPI-HBP1), a facilitator of lipoprotein lipase, lead to severely elevated triglycerides (87). Together with previous evidence (9), the above mentioned findings from 2017 suggest that pharmacological improved lipoprotein lipase activity, directly or through blocking inhibitors of the enzyme, will lead to lower triglycerides and lower ASCVD risk.
Another novel observation is that triglyceride-related genetic variant were associated with mitral annular calcification (88); future studies should examine if lowering of triglycerides will reduce mitral valve disease. Finally, as many guidelines worldwide now recommend non-fasting rather than fasting lipid profiles the average triglyceride levels during most of a 24 h cycle will be obvious for many patients and clinicians in the future (89).
High-density lipoprotein cholesterol
Low HDL cholesterol is considered a risk marker (not a causal factor) for ASCVD. Previously, it was thought that high HDL cholesterol would prevent or help reverse atherosclerosis by mediating transfer of cholesterol from the arterial wall to the liver for excretion. Cholesteryl ester transfer protein inhibitors increase the concentration of HDL cholesterol by blocking cholesterol transfer between HDL and other lipoprotein particles, and not necessarily through cholesterol uptake from the arterial wall.
Results of recent trials showed that treatment with CETP inhibitors increased HDL cholesterol in the ACCELERATE trial (90) and in the dal-OUTCOMES study (91), without profound reductions of apolipoprotein B, and had no effect on ASCVD (Figure 3); the Dal-GenE randomized trial is ongoing to examine cardiovascular effects of dalcetrapib in a genetically defined population (92). In the ILLUMINATE trial with 72% higher HDL cholesterol increases in ASCVD and all-cause mortality was observed (93); the negative outcome in ILLUMINATE have been associated with off-target effects. Although the recent REVEAL HPS-3/TIMI-55 study observed 9% less ASCVD (absolute risk reduction 1.0%) coinciding with 104% higher HDL cholesterol, a reduction in apolipoprotein B containing lipoproteins more likely explains the beneficial effects,4 as also supported by a genetic Mendelian randomization study (94). None of the CETP inhibitors will be available for clinical practice.

Importantly, current ESC/EAS dyslipidaemia guidelines do not recommend HDL cholesterol as a treatment target in ASCVD prevention (2).
Importantly, the cardiovascular benefit of anacetrapib should not be compared directly to that of statins, ezetimibe, or PCSK9 inhibitors, all working mainly through up-regulation of LDL receptors to reduce LDL cholesterol. In contrast, CETP inhibition influence levels of cholesterol in LDL, other apolipoprotein B containing lipoproteins and HDL through exchange of cholesterol and triglycerides between lipoprotein particles.
Notably, it has previously been suggested that vascular effects of HDL are altered in patients with ASCVD and chronic kidney disease, in part due to alterations of the protein cargo and small molecules such as symmetric dimethylarginine (95). These findings are now further supported by recent data, indicating that in patients with high symetric dimethylarginine levels increased HDL cholesterol is associated with adverse cardiovascular outcomes (96).
Moreover, in a large-scale analysis from two Copenhagen prospective population-based studies, it was observed that men and women in the general population with extreme high HDL cholesterol paradoxically have high all-cause mortality (97) (Figure 4); this further indicates that high HDL cholesterol levels have to be interpreted with caution and are not necessarily beneficial. Certainly, at high HDL cholesterol concentrations the HDL particle may not be functioning properly.

Inflammation
Inflammation plays a critical role in atherosclerosis and ASCVD (98), as well as in cancer (99). Intra-arterial accumulated lipoproteins initiate and modulate low grade inflammation and the production of cytokines and C-reactive protein. However, even after aggressive treatment and control of LDL cholesterol with statins, there remains residual risk. This residual inflammation and residual risk was addressed by the recent landmark CANTOS trial.
The CANTOS trial enrolled 10,061 patients with previous myocardial infarction and C-reactive protein ≥2 mg/L despite the use of aggressive secondary prevention strategies. Findings of this study include that anti-inflammatory therapy targeting interleukin-1β with canakinumab in the highest dose reduced ASCVD by 14% (absolute risk reduction ≈2%), total cancer mortality by 51% (absolute risk reduction ≈2.5%), and lung cancer incidence by 67% (absolute risk reduction ≈1.6%) (5, 100). Adverse events included a small absolute increase in fatal infection or sepsis, but also a beneficial effect on osteoarthritis and gout. The cardiovascular benefit obtained at the 150 mg dose of canakinumab came at the expense of an excess of mortality from infection, implying a narrow therapeutic window for such an anti-inflammatory approach.
In a follow-up study, it was suggested that the magnitude of reduction in C-reactive protein following a single dose of canakinumab might provide a simple clinical method to identify individuals most likely to accrue the largest benefit from continued treatment (101). These data further suggested that lower is better for inflammation reduction with canakinumab.
It is yet unclear if and when canakinumab will be available for ASCVD prevention. However, it is also important to recognize the value of clinical trial results like those from CANTOS for mechanistic insight in development of myocardial infarction (102). For example could such data of the vascular impact of targeting inflammation help us better understand the relative role of plaque rupture and plaque erosion in myocardial infarction?
Importantly, substantial residual ASCVD risk remains even after optimal lifestyle and medical therapy according to ESC/EAS Guidelines (1, 2), as well as after additional canakinumab therapy (5), lipid-lowering therapy with the cholesterol absorption inhibitors ezetimibe (103), the PCSK9 inhibitors evolocumab (3) and bococizumab (48), bezafibrate (104), or with the CETP inhibitor anacetrapib (4). Therefore, there remain large unmet medical needs for ASCVD prevention.
Diabetes
A paradigm shift in the management of type 2 diabetes (T2D) has been observed with sodium/glucose cotransporter 2 inhibitors (SGLT-2i). This class of agents prevent re-absorption of glucose from the urine, thus resulting in glycosuria and lower blood glucose levels. It seems however that they possess additional, yet not fully identified effects that enhance their protective effect on the cardiovascular system. These drugs reduce the risk of cardiovascular complications, kidney disease, and death beyond glycaemic control (105–107), benefits that recently were confirmed in 10,142 patients with T2D and high cardiovascular risk from CANVAS and CANVAS-Renal trials designed to assess effects on albuminuria (108); canagliflozin also lowered progression of albuminuria and loss of kidney function. In consideration of the increased risk of amputations seen, care is warranted in the use of canagliflozin in patients at such risk (108). Similarly, liraglutide, a glucagon like peptide 1 (GLP1) receptor agonist, reduced not only ASCVD (109) but also development and progression of diabetic kidney disease (110).
Further, CVD-REAL, a large observational study of T2D patients of 15% with and 85% without established ASCVD, found that the SGLT-2i drug class was associated with a lower risk of heart failure and all-cause mortality (111). The CVD-REAL Nordic – thanks to a complete population-level registries in Denmark, Norway, and Sweden – demonstrated that SGLT-2i also were associated with reduced cardiovascular mortality and morbidity (112). These retrospective cohort studies extend the results of EMPA-REG OUTCOME (106) and CANVAS (108) to the unselected T2D population. A putative confirmation of these results by the ongoing trials such as DECLARE-TIMI 58 (dapagliflozin; NCT01730534), due in 2019, would certainly impact clinical practice in primary prevention.
In patients with insulin resistance (but not diabetes) and a history of cerebrovascular accidents, the thiazolidinedione drug pioglitazone in the IRIS trial showed a 24% reduction of ASCVD (absolute risk reduction 2.8%) and lower progression to diabetes (113), reinforcing the emerging precision-medicine approaches to vascular disease and that pioglitazone may represent an option for secondary prevention in selected patients with cerebrovascular disease. Furthermore, the TOSCA IT trial showed cardiovascular safety of second-line glucose lowering drugs (114); the study examined effects of add-on pioglitazone vs. sulfonylureas on the incidence of ASCVD events in patients inadequately glucose-controlled with metformin and was stopped early because of futility. The confirmed safety profile in combination with the wide affordability of pioglitazone and sulfonylureas might promote trials comparing outcomes with ‘newer’ glucose-lowering drugs.
The EXSCEL trial recruited the hitherto largest patient population of any cardiovascular outcomes trial of the GLP1 receptor agonist class with more than 14 500 patients across 35 countries (115). In patients with T2D at a wide range of cardiovascular risk, exenatide extended-release once weekly compared with placebo showed cardiovascular safety. Although the primary efficacy objective of ASCVD events missed statistical significance, nominally 11.4% ASCVD events were observed in the exenatide vs. 12.2% in the placebo arm. Additional information from ongoing studies are awaited to define the effects of specific drugs, provide further insights into the mechanisms of cardiovascular benefit and put these results in the perspective of current treatment algorithms and healthcare economy.
In patients with T2D and high ASCVD risk, the long-acting insulin degludec compared with basal insulin glargine caused 40% fewer severe hypoglycaemic events and was non-inferior with respect to ASCVD (116). Further, intensive lifestyle intervention in patients with T2D lead to reduced use of glucose-lowering medication, but not to better glycaemic control (117).
Interestingly, although diabetes risk was not increased during short-term therapy with PCSK9 inhibitors (3, 48) lifelong genetically reduced PCSK9 and corresponding lower LDL cholesterol did appear to cause a small increase in risk of diabetes, but only in those with impaired fasting glucose (41). Also, genetic evidence document that overweight and obesity, either through increased body mass index or wait-to-hip ratio, is causally related to increased risk of both diabetes and ASCVD (118–121). Finally, in a healthy Asian population without comorbidities impaired fasting glucose and prehypertension were important risk factors for atrial fibrillation (122).
Hypertension
Poor medication adherence and late initiation of blood pressure (BP)-lowering represent important but missed opportunities for cardiovascular prevention. Previous studies had reported BP-lowering following catheter ablation for renal denervation (123). However, the large SYMPLICITY HTN-3 trial did not confirm these findings (124), possibly due to insufficient ablation, adherence to antihypertensive therapy and/or patient selection (125). Therefore, SPYRAL HTN-OFF MED randomized drug-naive or drug-discontinued hypertensive patients to more extensive and more distal denervation of renal arteries, in a blinded design including a sham procedure in controls and drug testing for patient compliance (125). Office systolic BP decreased by 10 mmHg and 24 h ambulatory BP by 6 mmHg, without major adverse events. Although these new data on renal denervation are interesting, they should be interpreted cautiously in light of the prior failures in this field. Nevertheless, these results with rigorous sham-design set a new standard for future interventional and surgical clinical studies.
Substantial BP-lowering could potentially cause severe side effects, like in the SPRINT trial of individuals who received intensive BP-lowering of 15 mmHg and consequently, benefitted from reduced cardiovascular events and mortality (126); however, these individuals reported similar physical, mental, and depressive outcomes compared with those receiving standard treatment (127). Also, in meta-analyses of hypertensive patients ≥65 years intensive BP-lowering reduced cardiovascular disease, cardiovascular mortality, and heart failure, but increased renal failure (128). Finally, a recent population-based study documented transgenerational risk of hypertension from grandparents through parents to grandchildren (129).
Arterial and venous thrombosis
Arterial thrombosis, especially in the coronary arteries, represents the most common precipitant of acute vascular syndromes, such as myocardial infarction and limb ischaemia. The Cardiovascular Outcomes for People Using Anticoagulation Strategies (COMPASS) trial assigned 27,395 patients with stable atherosclerotic vascular disease to receive rivaroxaban plus aspirin, rivaroxaban alone, or aspirin alone (6). The study was prematurely stopped due to 24% fewer ASCVD events (absolute risk reduction 1.3%) and 18% fewer deaths (absolute risk reduction 0.7%) in the rivaroxaban-plus-aspirin group compared with the aspirin-alone group; however, this was at the cost of 70% increased major bleeds (absolute risk increase 1.2%). Younger individuals compared with the elderly showed relatively larger reduction of ASCVD and lower bleeding risk. The effects of proton pump inhibitors on bleeds that are tested in a partial factorial design are pending. It is unclear how very low dose rivaroxaban + aspirin would compare with dual antiplatelet therapy or to the combination of rivaroxaban + P2Y12 inhibitor. In the COMPASS subgroup of 27% with chronic peripheral arterial disease, rivaroxaban plus aspirin vs. aspirin alone in addition reduced amputations (130). Since evidence-based treatments for patients with
peripheral arterial disease are scarce, low-dose rivaroxaban provides an important novel strategy for this high-risk population.
In patients with acute coronary syndrome dual antiplatelet therapy of low-dose rivaroxaban or aspirin, in combination with clopidogrel or ticagrelor, lead to similar risk of bleeding in 5% of patients (131). Importantly, discontinuation of any direct oral anticoagulant 3 days prior to elective invasive procedures will secure minimal concentrations pre-procedure in almost all patients (132).
A Danish study randomized >50,000 men aged 65–74 to screening or not for abdominal aortic aneurism, peripheral arterial disease, and hypertension; those diagnosed in the screening group were offered relevant follow-up and treatment including surgery and antihypertensive medication, which was associated with a 7% reduced all-cause mortality (absolute risk reduction 0.6%) primarily linked to initiation of pharmacological therapy (133). Importantly, mortality related to abdominal aortic aneurism may differ from country to country, and can be influenced by rate of surgical repair and aneurysm diameter at repair (134). A low ankle-brachial index help identify patients with abdominal aortic aneurism and peripheral arterial disease and predict ASCVD events, although to a lesser extent than increased coronary artery calcification (135).
Arterial thrombosis depends on atherosclerotic plaque vulnerability, which likely differs in individuals taking statins or not due to reduced lipid-driven plaque inflammation in those on statins (136). Interestingly, new data support that a chronically affected haematopoietic system potentially drive low-grade inflammation in patients with atherosclerosis (137).
For venous thromboembolism, meta-analyses of observational studies found a 27% reduced risk of recurrent venous thromboembolism associated with statin use (138), in accordance with findings in the randomized JUPITER trial(139). Finally, in patients with venous thromboembolism in equipoise for continued anticoagulation, the risk of a recurrent event was reduced approximately 70% by rivaroxaban compared with aspirin, without a significant increase in bleeding rates (140); this confirms previous studies with other novel oral anticoagulants.
Guidelines and consensus statements
Despite evidence-based recommendation for widespread use of statins in both primary and secondary prevention of ASCVD (1, 2), statin compliance is a major problem worldwide (141, 142), partly due to negative press (143, 144) and in consequence discontinuation of statin use and increased risk of myocardial infarction and cardiovascular mortality (143–146). In support, in the ASCOT-LLA trial muscle-related adverse events were similar in those receiving atorvastatin and placebo during blinding, however, after un-blinding and follow-up for an additional 2.3 years muscle-related adverse events were now 41% higher in those who knew they were receiving atorvastatin (147). Therefore, any patient claiming statin intolerance including muscle symptoms needs careful counselling with his or her physician, including better diagnostics of statin intolerance and advice on how to continue statin therapy despite perceived side effects (141, 142, 148).
Various updates of major guidelines for prevention of cardiovascular disease has occurred lately (1, 149–154), and despite use of the same scientific evidence to guide lifestyle changes and medical intervention advise tend to differ between guidelines. For example, the ACC/AHA guidelines compared with the ESC/EAS guidelines placed higher priority for assigning statins in primary prevention to those who later developed ASCVD (155); this difference was mainly explained by the fact that the American guidelines assigned statin therapy to more individuals that the European guidelines. That said, the European guidelines is limited by using the SCORE algorithm for ASCVD risk assignment based only on ASCVD mortality in cohorts recruited many years ago, and limited to only 40–65 years old (156, 157). Although the risk of ASCVD increases with increasing age above 65 years (156) with age as the most important ASCVD risk predictor, arguments differ with respect to how important age should be in determining statin assignment (158, 159).
Although the American ACC/AHA risk score overestimates ASCVD risk, particularly in Chinese (160), the European ESC/EAS SCORE may in some populations overestimate risk even more (155). Therefore, ideally risk scores for ASCVD needs to be recalibrated to each country and ethnic group before it is used to assign statin therapy. In 2017, exactly that has happened for the UK QRISK3 risk prediction algorithms for the NICE guidelines (149), using current data from 981 general practices and 7.9 million patients aged 25–84 in England to develop new scores and another 328 practices and 2.7 million patients to validate the new score algorithms (161).
By 2017, the use of non-fasting rather than fasting lipid profiles is now recommended in many guidelines and consensus statements worldwide (89), including in the UK (149), Europe (1, 2, 162), Canada (150, 150) Brazil (163), and in the USA (153, 164, 165). Finally, new USA guidelines have lowered the threshold for the definition of hypertension to ≥130/80 mmHg systolic/diastolic BP (earlier 140/90 mmHg) (166), placing very large proportions of adult populations in potential need for BP-lowering medication or intensified BP-lowering in the USA.
Conclusion
2017 has been a very exciting year for studies in ASCVD prevention, including landmark clinical trials, genetic Mendelian randomization studies, and observational prospective cohort studies. Figure 1 illustrates some of the new concepts for additional preventive measures in secondary prevention in a patient with coronary heart disease already on statin, aspirin, ACE inhibitor, and beta-blocker. Naturally, many new concepts await confirmation by additional studies and their test in clinical practice.
Importantly, considerable inter-individual variability has been noted in the response to a number of the agents discussed in this review. Therefore, for all new (and old) drugs, it is important to monitor response, particularly at a time when economic pressures oblige clinicians to use therapeutic agents in an optimal manner on a personalised basis.
Conflict of interest: none declared.
References
1. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney MT, Corra U, Cosyns B, Deaton C, Graham I, Hall MS, Hobbs FD, Lochen ML, Lollgen H, Marques-Vidal P, Perk J, Prescott E, Redon J, Richter DJ, Sattar N, Smulders Y, Tiberi M, van der Worp HB, van DI, Verschuren WM. 2016 European guidelines on cardiovascular disease prevention in clinical practice: the sixth joint task force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of 10 societies and by invited experts) developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 2016; 37: 2315–2381.
2. Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, Hoes AW, Jennings CS, Landmesser U, Pedersen TR, Reiner Z, Riccardi G, Taskinen MR, Tokgozoglu L, Verschuren WM, Vlachopoulos C, Wood DA, Zamorano JL. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J 2016; 37: 2999–3058.
3. Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, Sever PS, Pedersen TR. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017; 376: 1713–1722.
4. Effects of anacetrapib in patients with atherosclerotic vascular disease. N Engl J Med 2017; 377: 1217–1227.
5. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017; 377: 1119–1131.
6. Eikelboom JW, Connolly SJ, Bosch J, Dagenais GR, Hart RG, Shestakovska O, Diaz R, Alings M, Lonn EM, Anand SS, Widimsky P, Hori M, Avezum A, Piegas LS, Branch KRH, Probstfield J, Bhatt DL, Zhu J, Liang Y, Maggioni AP, Lopez-Jaramillo P, O’Donnell M, Kakkar AK, Fox KAA, Parkhomenko AN, Ertl G, Störk S, Keltai M, Ryden L, Pogosova N, Dans AL, Lanas F, Commerford PJ, Torp-Pedersen C, Guzik TJ, Verhamme PB, Vinereanu D, Kim J-H, Tonkin AM, Lewis BS, Felix C, Yusoff K, Steg PG, Metsarinne KP, Cook Bruns N, Misselwitz F, Chen E, Leong D, Yusuf S. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med 2017; 377: 1319–1330.
7. Timmis A, Townsend N, Gale C, Grobbee R, Maniadakis N, Flather M, Wilkins E, Wright L, Vos R, Bax J, Blum M, Pinto F, Vardas P. European Society of Cardiology: cardiovascular disease statistics 2017. Eur Heart J; doi: https://doi.org/10.1093/eurheartj/ehx628. Published online ahead of print 27 November 2017.
8. Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey SG. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med 2008; 27: 1133–1163.
9. Nordestgaard BG. Triglyceride-rich lipoproteins and atherosclerotic cardiovascular disease: new insights from epidemiology, genetics, and biology. Circ Res 2016; 118: 547–563.
10. Davey SG, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet 2014; 23: R89–R98.
11. Lassale C, Tzoulaki I, Moons KGM, Sweeting M, Boer J, Johnson L, Huerta JM, Agnoli C, Freisling H, Weiderpass E, Wennberg P, van der AD, Arriola L, Benetou V, Boeing H, Bonnet F, Colorado-Yohar SM, Engstrom G, Eriksen AK, Ferrari P, Grioni S, Johansson M, Kaaks R, Katsoulis M, Katzke V, Key TJ, Matullo G, Melander O, Molina-Portillo E, Moreno-Iribas C, Norberg M, Overvad K, Panico S, Quiros JR, Saieva C, Skeie G, Steffen A, Stepien M, Tjonneland A, Trichopoulou A, Tumino R, van der Schouw YT, Verschuren WMM, Langenberg C, Di AE, Riboli E, Wareham NJ, Danesh J, Butterworth AS. Separate and combined associations of obesity and metabolic health with coronary heart disease: a pan-European case-cohort analysis. Eur Heart J; doi: https://doi.org/10.1093/eurheartj/ehx448. Published online 14 August 2017.
12. Thomsen M, Nordestgaard BG. Myocardial infarction and ischemic heart disease in overweight and obesity with and without metabolic syndrome. JAMA Intern Med 2014; 174: 15–22.
13. Hansen L, Netterstrøm MK, Johansen NB, Rønn PF, Vistisen D, Husemoen LLN, Jørgensen ME, Rod NH, Færch K. Metabolically healthy obesity and ischemic heart disease: a 10-year follow-up of the Inter99 study. J Clin Endocrinol Metab 2017; 102: 1934–1942.
14. Mongraw-Chaffin M, Foster MC, Kalyani RR, Vaidya D, Burke GL, Woodward M, Anderson CA. Obesity severity and duration are associated with incident metabolic syndrome: evidence against metabolically healthy obesity from the multi-ethnic study of atherosclerosis. J Clin Endocrinol Metab 2016; 101: 4117–4124.
15. Caleyachetty R, Thomas GN, Toulis KA, Mohammed N, Gokhale KM, Balachandran K, Nirantharakumar K. Metabolically healthy obese and incident cardiovascular disease events among 3.5 million men and women. J Am Coll Cardiol 2017; 70: 1429–1437.
16. Bangalore S, Fayyad R, Laskey R, DeMicco DA, Messerli FH, Waters DD. Body-weight fluctuations and outcomes in coronary disease. N Engl J Med 2017; 376: 1332–1340.
17. Gunter MJ, Murphy N, Cross AJ, Dossus L, Dartois L, Fagherazzi G, Kaaks R, Kuhn T, Boeing H, Aleksandrova K, Tjonneland A, Olsen A, Overvad K, Larsen SC, Redondo Cornejo ML, Agudo A, Sanchez Perez MJ, Altzibar JM, Navarro C, Ardanaz E, Khaw KT, Butterworth A, Bradbury KE, Trichopoulou A, Lagiou P, Trichopoulos D, Palli D, Grioni S, Vineis P, Panico S, Tumino R, Bueno-de-Mesquita B, Siersema P, Leenders M, Beulens JWJ, Uiterwaal CU, Wallstrom P, Nilsson LM, Landberg R, Weiderpass E, Skeie G, Braaten T, Brennan P, Licaj I, Muller DC, Sinha R, Wareham N, Riboli E. Coffee drinking and mortality in 10 European countries: a multinational cohort study. Ann Intern Med 2017; 167: 236–247.
18. Park SY, Freedman ND, Haiman CA, Le ML, Wilkens LR, Setiawan VW. Association of coffee consumption with total and cause-specific mortality among Nonwhite populations. Ann Intern Med 2017; 167: 228–235.
19. Nordestgaard AT, Nordestgaard BG. Coffee intake, cardiovascular disease and all-cause mortality: observational and Mendelian randomization analyses in 95 000-223 000 individuals. Int J Epidemiol 2016; 45: 1938–1952.
20. Ding M, Huang T, Bergholdt HK, Nordestgaard BG, Ellervik C, Qi L. Dairy consumption, systolic blood pressure, and risk of hypertension: Mendelian randomization study. BMJ 2017; 356: j1000.
21. Bergholdt HK, Nordestgaard BG, Varbo A, Ellervik C. Milk intake is not associated with ischaemic heart disease in observational or Mendelian randomization analyses in 98, 529 Danish adults. Int J Epidemiol 2015; 44: 587–603.
22. Brunner S, Herbel R, Drobesch C, Peters A, Massberg S, Kaab S, Sinner MF. Alcohol consumption, sinus tachycardia, and cardiac arrhythmias at the Munich Octoberfest: results from the Munich Beer Related Electrocardiogram Workup Study (MunichBREW). Eur Heart J 2017; 38: 2100–2106.
23. Bell S, Daskalopoulou M, Rapsomaniki E, George J, Britton A, Bobak M, Casas JP, Dale CE, Denaxas S, Shah AD, Hemingway H. Association between clinically recorded alcohol consumption and initial presentation of 12 cardiovascular diseases: population based cohort study using linked health records. BMJ 2017; 356: j909.
24. Xi B, Veeranki SP, Zhao M, Ma C, Yan Y, Mi J. Relationship of alcohol consumption to all-cause, cardiovascular, and cancer-related mortality in U.S. adults. J Am Coll Cardiol 2017; 70: 913–922.
25. Whitman IR, Agarwal V, Nah G, Dukes JW, Vittinghoff E, Dewland TA, Marcus GM. Alcohol abuse and cardiac disease. J Am Coll Cardiol 2017; 69: 13–24.
26. Tillmann T, Vaucher J, Okbay A, Pikhart H, Peasey A, Kubinova R, Pajak A, Tamosiunas A, Malyutina S, Hartwig FP, Fischer K, Veronesi G, Palmer T, Bowden J, Davey SG, Bobak M, Holmes MV. Education and coronary heart disease: Mendelian randomisation study. BMJ 2017; 358: j3542.
27. Celis-Morales CA, Lyall DM, Anderson J, Iliodromiti S, Fan Y, Ntuk UE, Mackay DF, Pell JP, Sattar N, Gill JM. The association between physical activity and risk of mortality is modulated by grip strength and cardiorespiratory fitness: evidence from 498 135 UK-Biobank participants. Eur Heart J 2017; 38: 116–122.
28. Lyall DM, Celis-Morales CA, Anderson J, Gill JM, Mackay DF, McIntosh AM, Smith DJ, Deary IJ, Sattar N, Pell JP. Associations between single and multiple cardiometabolic diseases and cognitive abilities in 474 129 UK Biobank participants. Eur Heart J 2017; 38: 577–583.
29. Lv J, Yu C, Guo Y, Bian Z, Yang L, Chen Y, Tang X, Zhang W, Qian Y, Huang Y, Wang X, Chen J, Chen Z, Qi L, Li L, Chen J, Chen Z, Collins R, Li L, Peto R, Avery D, Boxall R, Bennett D, Chang Y, Chen Y, Chen Z, Clarke R, Du H, Gilbert S, Hacker A, Holmes M, Iona A, Kartsonaki C, Kerosi R, Kong L, Kurmi O, Lancaster G, Lewington S, Lin K, McDonnell J, Mei W, Millwood I, Nie Q, Radhakrishnan J, Rafiq S, Ryder P, Sansome S, Schmidt D, Sherliker P, Sohoni R, Turnbull I, Walters R, Wang J, Wang L, Yang L, Yang X, Bian Z, Chen G, Guo Y, Han B, Hou C, Lv J, Pei P, Qu S, Tan Y, Yu C, Zhou H, Pang Z, Gao R, Wang S, Liu Y-M, Du RR, Zang Y, Cheng L, Tian X, Zhang H, Lv S, Wang J, Hou W, Yin J, Jiang G, Zhou X, Yang L, He H, Yu B, Li Y, Mu H, Xu Q, Dou M, Ren J, Wang S, Hu X, Wang H, Chen J, Fu Y, Fu Z, Wang X, Weng M, Zheng X, Li Y, Li H, Wang Y, Wu M, Zhou J, Tao R, Yang J, Ni C, Zhang J, Hu Y, Lu Y, Ma L, Tang A, Zhang S, Jin J, Liu J, Tang Z, Chen N, Huang Y, Li M, Meng J, Pan R, Jiang Q, Zhang W, Liu Y, Wei L, Zhou L, Chen N, Guan H, Wu X, Zhang N, Chen X, Tang X, Luo G, Li J, Chen X, Zhong X, Liu J, Sun Q, Ge P, Ren X, Dong C, Zhang H, Mao E, Wang X, Wang T, Zhang X, Zhang D, Zhou G, Feng S, Chang L, Fan L, Gao Y, He T, Sun H, He P, Hu C, Lv Q, Zhang X, Yu M, Hu R, Wang H, Qian Y, Wang C, Xie K, Chen L, Zhang Y, Pan D, Huang Y, Chen B, Yin L, Jin D, Liu H, Fu Z, Xu Q, Xu X, Zhang H, Xiong Y, Long H, Li X, Zhang L, Qiu Z. Adherence to healthy lifestyle and cardiovascular diseases in the Chinese population. J Am Coll Cardiol 2017; 69: 1116–1125.
30. Uzhova I, Fuster V, Fernández-Ortiz A, Ordovás JM, Sanz J, Fernández-Friera L, López-Melgar B, Mendiguren JM, Ibáñez B, Bueno H, Peñalvo JL. The importance of breakfast in atherosclerosis disease: insights from the PESA study. J Am Coll Cardiol 2017; 70: 1833–1842.
31. Minneboo M, Lachman S, Snaterse M, Jørstad HT, ter Riet G, Boekholdt SM, Scholte Op Reimer WJM, Peters RJG, Riezebos RK, van Liebergen RAM, van der Spank A, van Dantzig JM, de Milliano PAR, van Hessen MWJ, Kragten JA, Jaarsma W, den Hartog FR, Bartels GL, Aengevaeren WRM, van Rossum P, Anneveldt A, de Vries CJ. Community-based lifestyle intervention in patients with coronary artery disease: the RESPONSE-2 trial. J Am Coll Cardiol 2017; 70: 318–327.
32. Miller V, Mente A, Dehghan M, Rangarajan S, Zhang X, Swaminathan S, Dagenais G, Gupta R, Mohan V, Lear S, Bangdiwala SI, Schutte AE, Wentzel-Viljoen E, Avezum A, Altuntas Y, Yusoff K, Ismail N, Peer N, Chifamba J, Diaz R, Rahman O, Mohammadifard N, Lana F, Zatonska K, Wielgosz A, Yusufali A, Iqbal R, Lopez-Jaramillo P, Khatib R, Rosengren A, Kutty VR, Li W, Liu J, Liu X, Yin L, Teo K, Anand S, Yusuf S. Fruit, vegetable, and legume intake, and cardiovascular disease and deaths in 18 countries (PURE): a prospective cohort study. Lancet 2017; 390: 2037–2049.
33. Munzel T, Sorensen M, Gori T, Schmidt FP, Rao X, Brook J, Chen LC, Brook RD, Rajagopalan S. Environmental stressors and cardio-metabolic disease: part I-epidemiologic evidence supporting a role for noise and air pollution and effects of mitigation strategies. Eur Heart J 2017; 38: 550–556.
34. Munzel T, Sorensen M, Gori T, Schmidt FP, Rao X, Brook FR, Chen LC, Brook RD, Rajagopalan S. Environmental stressors and cardio-metabolic disease: part II-mechanistic insights. Eur Heart J 2017; 38: 557–564.
35. Cai Y, Hansell AL, Blangiardo M, Burton PR, de HK, Doiron D, Fortier I, Gulliver J, Hveem K, Mbatchou S, Morley DW, Stolk RP, Zijlema WL, Elliott P, Hodgson S. Long-term exposure to road traffic noise, ambient air pollution, and cardiovascular risk factors in the HUNT and lifelines cohorts. Eur Heart J 2017; 38: 2290–2296.
36. Fuks KB, Weinmayr G, Basagana X, Gruzieva O, Hampel R, Oftedal B, Sorensen M, Wolf K, Aamodt G, Aasvang GM, Aguilera I, Becker T, Beelen R, Brunekreef B, Caracciolo B, Cyrys J, Elosua R, Eriksen KT, Foraster M, Fratiglioni L, Hilding A, Houthuijs D, Korek M, Kunzli N, Marrugat J, Nieuwenhuijsen M, Ostenson CG, Penell J, Pershagen G, Raaschou-Nielsen O, Swart WJR, Peters A, Hoffmann B. Long-term exposure to ambient air pollution and traffic noise and incident hypertension in seven cohorts of the European study of cohorts for air pollution effects (ESCAPE). Eur Heart J 2017; 38: 983–990.
37. Cohen AJ, Brauer M, Burnett R, Anderson HR, Frostad J, Estep K, Balakrishnan K, Brunekreef B, Dandona L, Dandona R, Feigin V, Freedman G, Hubbell B, Jobling A, Kan H, Knibbs L, Liu Y, Martin R, Morawska L, Pope CAIII, Shin H, Straif K, Shaddick G, Thomas M, van DR, van DA, Vos T, Murray CJL, Forouzanfar MH. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the global burden of diseases study 2015. Lancet 2017; 389: 1907–1918.
38. Ference BA, Ginsberg HN, Graham I, Ray KK, Packard CJ, Bruckert E, Hegele RA, Krauss RM, Raal FJ, Schunkert H, Watts GF, Boren J, Fazio S, Horton JD, Masana L, Nicholls SJ, Nordestgaard BG, van de Sluis B, Taskinen MR, Tokgozoglu L, Landmesser U, Laufs U, Wiklund O, Stock JK, Chapman MJ, Catapano AL. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J 2017; 38: 2459–2472.
39. Kaplan H, Thompson RC, Trumble BC, Wann LS, Allam AH, Beheim B, Frohlich B, Sutherland ML, Sutherland JD, Stieglitz J, Rodriguez DE, Michalik DE, Rowan CJ, Lombardi GP, Bedi R, Garcia AR, Min JK, Narula J, Finch CE, Gurven M, Thomas GS. Coronary atherosclerosis in indigenous South American Tsimane: a cross-sectional cohort study. Lancet 2017; 389: 1730–1739.
40. Giugliano RP, Pedersen TR, Park JG, Ferrari GM, Gaciong ZA, Ceska R, Toth K, Gouni-Berthold I, Lopez-Miranda J, Schiele F, Mach F, Ott BR, Kanevsky E, Pineda AL, Somaratne R, Wasserman SM, Keech AC, Sever PS, Sabatine MS. Clinical efficacy and safety of achieving very low LDL-cholesterol concentrations with the PCSK9 inhibitor evolocumab: a prespecified secondary analysis of the FOURIER trial. Lancet 2017; 390: 1962–1971.
41. Ference BA, Robinson JG, Brook RD, Catapano AL, Chapman MJ, Neff DR, Voros S, Giugliano RP, Davey SG, Fazio S, Sabatine MS. Variation in PCSK9 and HMGCR and risk of cardiovascular disease and diabetes. N Engl J Med 2016; 375: 2144–2153.
42. Giugliano RP, Mach F, Zavitz K, Kurtz C, Im K, Kanevsky E, Schneider J, Wang H, Keech A, Pedersen TR, Sabatine MS, Sever PS, Robinson JG, Honarpour N, Wasserman SM, Ott BR. Cognitive function in a randomized trial of evolocumab. N Engl J Med 2017; 377: 633–643.
43. Benn M, Nordestgaard BG, Frikke-Schmidt R, Tybjaerg-Hansen A. Low LDL cholesterol, PCSK9 and HMGCR genetic variation, and risk of Alzheimer’s disease and Parkinson’s disease: Mendelian randomisation study. BMJ 2017; 357: j1648.
44. Robinson JG, Rosenson RS, Farnier M, Chaudhari U, Sasiela WJ, Merlet L, Miller K, Kastelein JJ. Safety of very low low-density lipoprotein cholesterol levels with alirocumab: pooled data from randomized trials. J Am Coll Cardiol 2017; 69: 471–482.
45. Toth PP, Descamps O, Genest J, Sattar N, Preiss D, Dent R, Djedjos C, Wu Y, Geller M, Uhart M, Somaratne R, Wasserman SM. Pooled safety analysis of evolocumab in over 6000 patients from double-blind and open-label extension studies. Circulation 2017; 135: 1819–1831.
46. Giugliano RP, Wiviott SD, Blazing MA, De Ferrari GM, Park JG, Murphy SA, White JA, Tershakovec AM, Cannon CP, Braunwald E. Long-term safety and efficacy of achieving very low levels of low-density lipoprotein cholesterol: a prespecified analysis of the IMPROVE-IT trial. JAMA Cardiol 2017; 2: 547–555.
47. Ridker PM, Tardif JC, Amarenco P, Duggan W, Glynn RJ, Jukema JW, Kastelein JJP, Kim AM, Koenig W, Nissen S, Revkin J, Rose LM, Santos RD, Schwartz PF, Shear CL, Yunis C. Lipid-reduction variability and antidrug-antibody formation with bococizumab. N Engl J Med 2017; 376: 1517–1526.
48. Ridker PM, Revkin J, Amarenco P, Brunell R, Curto M, Civeira F, Flather M, Glynn RJ, Gregoire J, Jukema JW, Karpov Y, Kastelein JJP, Koenig W, Lorenzatti A, Manga P, Masiukiewicz U, Miller M, Mosterd A, Murin J, Nicolau JC, Nissen S, Ponikowski P, Santos RD, Schwartz PF, Soran H, White H, Wright RS, Vrablik M, Yunis C, Shear CL, Tardif JC. Cardiovascular efficacy and safety of bococizumab in high-risk patients. N Engl J Med 2017; 376: 1527–1539.
49. Landmesser U, Chapman MJ, Stock JK, Amarenco P, Belch JJF, Boren J, Farnier M, Ference BA, Gielen S, Graham I, Grobbee DE, Hovingh GK, Luscher TF, Piepoli MF, Ray KK, Stroes ES, Wiklund O, Windecker S, Zamorano JL, Pinto F, Tokgozoglu L, Bax JJ, Catapano AL. 2017 update of ESC/EAS task force on practical clinical guidance for proprotein convertase subtilisin/kexin type 9 inhibition in patients with atherosclerotic cardiovascular disease or in familial hypercholesterolaemia. Eur Heart J; doi: https://doi.org/10.1093/eurheartj/ehx549. Published online 16 October 2017.
50. Fitzgerald K, White S, Borodovsky A, Bettencourt BR, Strahs A, Clausen V, Wijngaard P, Horton JD, Taubel J, Brooks A, Fernando C, Kauffman RS, Kallend D, Vaishnaw A, Simon A. A highly durable RNAi therapeutic inhibitor of PCSK9. N Engl J Med 2017; 376: 41–51.
51. Ray KK, Landmesser U, Leiter LA, Kallend D, Dufour R, Karakas M, Hall T, Troquay RP, Turner T, Visseren FL, Wijngaard P, Wright RS, Kastelein JJ. Inclisiran in patients at high cardiovascular risk with elevated LDL cholesterol. N Engl J Med 2017; 376: 1430–1440.
52. Landlinger C, Pouwer MG, Juno C, van der Hoorn JWA, Pieterman EJ, Jukema JW, Staffler G, Princen HMG, Galabova G. The AT04A vaccine against proprotein convertase subtilisin/kexin type 9 reduces total cholesterol, vascular inflammation, and atherosclerosis in APOE*3Leiden.CETP mice. Eur Heart J 2017; 38: 2499–2507.
53. Hagiwara N, Kawada-Watanabe E, Koyanagi R, Arashi H, Yamaguchi J, Nakao K, Tobaru T, Tanaka H, Oka T, Endoh Y, Saito K, Uchida T, Matsui K, Ogawa H. Low-density lipoprotein cholesterol targeting with pitavastatin + ezetimibe for patients with acute coronary syndrome and dyslipidaemia: the HIJ-PROPER study, a prospective, open-label, randomized trial. Eur Heart J 2017; 38: 2264–2276.
54. Sjouke B, Kusters DM, Kindt I, Besseling J, Defesche JC, Sijbrands EJ, Roeters van Lennep JE, Stalenhoef AF, Wiegman A, de GJ, Fouchier SW, Kastelein JJ, Hovingh GK. Homozygous autosomal dominant hypercholesterolaemia in the Netherlands: prevalence, genotype-phenotype relationship, and clinical outcome. Eur Heart J 2015; 36: 560–565.
55. Benn M, Watts GF, Tybjærg-Hansen A, Nordestgaard BG. Mutations causative of familial hypercholesterolaemia: screening of 98 098 individuals from the Copenhagen general population study estimated a prevalence of 1 in 217. Eur Heart J 2016; 37: 1384–1394.
56. Wald DS, Bestwick JP, Morris JK, Whyte K, Jenkins L, Wald NJ. Child-parent familial hypercholesterolemia screening in primary care. N Engl J Med 2016; 375: 1628–1637.
57. Nordestgaard BG, Chapman MJ, Humphries SE, Ginsberg HN, Masana L, Descamps OS, Wiklund O, Hegele RA, Raal FJ, Defesche JC, Wiegman A, Santos RD, Watts GF, Parhofer KG, Hovingh GK, Kovanen PT, Boileau C, Averna M, Boren J, Bruckert E, Catapano AL, Kuivenhoven JA, Pajukanta P, Ray K, Stalenhoef AF, Stroes E, Taskinen MR, Tybjaerg-Hansen A. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur Heart J 2013; 34: 3478–3490a.
58. Cuchel M, Bruckert E, Ginsberg HN, Raal FJ, Santos RD, Hegele RA, Kuivenhoven JA, Nordestgaard BG, Descamps OS, Steinhagen-Thiessen E, Tybjærg-Hansen A, Watts GF, Averna M, Boileau C, Borén J, Catapano AL, Defesche JC, Hovingh GK, Humphries SE, Kovanen PT, Masana L, Pajukanta P, Parhofer KG, Ray KK, Stalenhoef AFH, Stroes E, Taskinen M-R, Wiegman A, Wiklund O, Chapman MJ. Homozygous familial hypercholesterolaemia: new insights and guidance for clinicians to improve detection and clinical management. A position paper from the Consensus Panel on Familial Hypercholesterolaemia of the European Atherosclerosis Society. Eur Heart J 2014; 35: 2146–2157.
59. Wiegman A, Gidding SS, Watts GF, Chapman MJ, Ginsberg HN, Cuchel M, Ose L, Averna M, Boileau C, Borén J, Bruckert E, Catapano AL, Defesche JC, Descamps OS, Hegele RA, Hovingh GK, Humphries SE, Kovanen PT, Kuivenhoven JA, Masana L, Nordestgaard BG, Pajukanta P, Parhofer KG, Raal FJ, Ray KK, Santos RD, Stalenhoef AFH, Steinhagen- Thiessen E, Stroes ES, Taskinen M-R, Tybjærg-Hansen A, Wiklund O. Familial hypercholesterolaemia in children and adolescents: gaining decades of life by optimizing detection and treatment. Eur Heart J 2015; 36: 2425–2437.
60. Tada H, Kawashiri MA, Nohara A, Inazu A, Mabuchi H, Yamagishi M. Impact of clinical signs and genetic diagnosis of familial hypercholesterolaemia on the prevalence of coronary artery disease in patients with severe hypercholesterolaemia. Eur Heart J 2017; 38: 1573–1579.
61. Nordestgaard BG, Benn M. Genetic testing for familial hypercholesterolaemia is essential in individuals with high LDL cholesterol: who does it in the world? Eur Heart J 2017; 38: 1580–1583.
62. Knowles JW, Rader DJ, Khoury MJ. Cascade screening for familial hypercholesterolemia and the use of genetic testing. JAMA 2017; 318: 381–382.
63. Kerr M, Pears R, Miedzybrodzka Z, Haralambos K, Cather M, Watson M, Humphries SE. Cost effectiveness of cascade testing for familial hypercholesterolaemia, based on data from familial hypercholesterolaemia services in the UK. Eur Heart J 2017; 38: 1832–1839.
64. Stoekenbroek RM, Kastelein JJP, Hovingh GK. Can we afford not to screen for FH? Eur Heart J 2017; 38: 1840–1842.
65. Besseling J, Reitsma JB, Gaudet D, Brisson D, Kastelein JJ, Hovingh GK, Hutten BA. Selection of individuals for genetic testing for familial hypercholesterolaemia: development and external validation of a prediction model for the presence of a mutation causing familial hypercholesterolaemia. Eur Heart J 2017; 38: 565–573.
66. Amor-Salamanca A, Castillo S, Gonzalez-Vioque E, Dominguez F, Quintana L, Lluis-Ganella C, Escudier JM, Ortega J, Lara-Pezzi E, Alonso-Pulpon L, Garcia-Pavia P. Genetically confirmed familial hypercholesterolemia in patients with acute coronary syndrome. J Am Coll Cardiol 2017; 70: 1732–1740.
67. Stein EA, Dann EJ, Wiegman A, Skovby F, Gaudet D, Sokal E, Charng MJ, Mohamed M, Luirink I, Raichlen JS, Sunden M, Carlsson SC, Raal FJ, Kastelein JJP. Efficacy of rosuvastatin in children with homozygous familial hypercholesterolemia and association with underlying genetic mutations. J Am Coll Cardiol 2017; 70: 1162–1170.
68. Braamskamp MJAM, Langslet G, McCrindle BW, Cassiman D, Francis GA, Gagne C, Gaudet D, Morrison KM, Wiegman A, Turner T, Miller E, Kusters DM, Raichlen JS, Martin PD, Stein EA, Kastelein JJP, Hutten BA. Effect of rosuvastatin on carotid intima-media thickness in children with heterozygous familial hypercholesterolemia: the CHARON study (hypercholesterolemia in children and adolescents taking rosuvastatin open label). Circulation 2017; 136: 359–366.
69. Bernelot Moens SJ, Neele AE, Kroon J, van der Valk FM, Van den Bossche J, Hoeksema MA, Hoogeveen RM, Schnitzler JG, Baccara-Dinet MT, Manvelian G, de Winther MPJ, Stroes ESG. PCSK9 monoclonal antibodies reverse the pro-inflammatory profile of monocytes in familial hypercholesterolaemia. Eur Heart J 2017; 38: 1584–1593.
70. Pérez de Isla L, Alonso R, Mata N, Fernández-Pérez C, Muñiz O, Díaz-Díaz JL, Saltijeral A, Fuentes-Jiménez F, de Andrés R, Zambón D, Piedecausa M, Cepeda JM, Mauri M, Galiana J, Brea Á, Sanchez Muñoz-Torrero JF, Padró T, Argueso R, Miramontes-González JP, Badimón L, Santos RD, Watts GF, Mata P. Predicting cardiovascular events in familial hypercholesterolemia: the SAFEHEART registry (Spanish familial hypercholesterolemia cohort study). Circulation 2017; 135: 2133–2144.
71. Langsted A, Kamstrup PR, Benn M, Tybjærg-Hansen A, Nordestgaard BG. High lipoprotein(a) as a possible cause of clinical familial hypercholesterolaemia: a prospective cohort study. Lancet Diabetes Endocrinol 2016; 4: 577–587.
72. Vallejo-Vaz A, Robertson M, Catapano A, Watts GF, Kastelein JJ, Packard CJ, Ford I, Ray KK. Low-density lipoprotein cholesterol lowering for the primary prevention of cardiovascular disease among men with primary elevations of low-density lipoprotein cholesterol levels of 190 mg/dL or above. Analyses from the WOSCOPS (West of Scotland coronary prevention study) 5-year randomized trial and 20-year observational follow-up. Circulation 2017; 136: 1878–1891.
73. Nordestgaard BG, Langsted A. Lipoprotein (a) as a cause of cardiovascular disease: insights from epidemiology, genetics, and biology. J Lipid Res 2016; 57: 1953–1975.
74. Gencer B, Kronenberg F, Stroes ES, Mach F. Lipoprotein(a): the revenant. Eur Heart J 2017; 38: 1553–1560.
75. Coassin S, Erhart G, Weissensteiner H, Eca Guimaraes de AM, Lamina C, Schonherr S, Forer L, Haun M, Losso JL, Kottgen A, Schmidt K, Utermann G, Peters A, Gieger C, Strauch K, Finkenstedt A, Bale R, Zoller H, Paulweber B, Eckardt KU, Huttenhofer A, Huber LA, Kronenberg F. A novel but frequent variant in LPA KIV-2 is associated with a pronounced Lp(a) and cardiovascular risk reduction. Eur Heart J 2017; 38: 1823–1831.
76. Tolbus A, Mortensen MB, Nielsen SF, Kamstrup PR, Bojesen SE, Nordestgaard BG. Kringle IV Type 2, not low lipoprotein(a), as a cause of diabetes: a novel genetic approach using SNPs associated selectively with lipoprotein(a) concentrations or with Kringle IV Type 2 repeats. Clin Chem 2017; 63: 1866–1876.
77. Tsimikas S. A test in context: lipoprotein(a): diagnosis, prognosis, controversies, and emerging therapies. J Am Coll Cardiol 2017; 69: 692–711.
78. Waldeyer C, Makarova N, Zeller T, Schnabel RB, Brunner FJ, Jorgensen T, Linneberg A, Niiranen T, Salomaa V, Jousilahti P, Yarnell J, Ferrario MM, Veronesi G, Brambilla P, Signorini SG, Iacoviello L, Costanzo S, Giampaoli S, Palmieri L, Meisinger C, Thorand B, Kee F, Koenig W, Ojeda F, Kontto J, Landmesser U, Kuulasmaa K, Blankenberg S. Lipoprotein(a) and the risk of cardiovascular disease in the European population: results from the BiomarCaRE consortium. Eur Heart J 2017; 38: 2490–2498.
79. Khan TZ, Hsu LY, Arai AE, Rhodes S, Pottle A, Wage R, Banya W, Gatehouse PD, Giri S, Collins P, Pennell DJ, Barbir M. Apheresis as novel treatment for refractory angina with raised lipoprotein(a): a randomized controlled cross-over trial. Eur Heart J 2017; 38: 1561–1569.
80. von Eckardstein A. Will you, nill you, I will treat you: the taming of lipoprotein(a). Eur Heart J 2017; 38: 1570–1572.
81. Varbo A, Ff JC, Wright RS. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med 2012; 367: 2089–2099.
82. Dewey FE, Gusarova V, Dunbar RL, O’Dushlaine C, Schurmann C, Gottesman O, McCarthy S, Van Hout CV, Bruse S, Dansky HM, Leader JB, Murray MF, Ritchie MD, Kirchner HL, Habegger L, Lopez A, Penn J, Zhao A, Shao W, Stahl N, Murphy AJ, Hamon S, Bouzelmat A, Zhang R, Shumel B, Pordy R, Gipe D, Herman GA, Sheu WHH, Lee I-T, Liang K-W, Guo X, Rotter JI, Chen Y-DI, Kraus WE, Shah SH, Damrauer S, Small A, Rader DJ, Wulff AB, Nordestgaard BG, Tybjærg-Hansen A, van den Hoek AM, Princen HMG, Ledbetter DH, Carey DJ, Overton JD, Reid JG, Sasiela WJ, Banerjee P, Shuldiner AR, Borecki IB, Teslovich TM, Yancopoulos GD, Mellis SJ, Gromada J, Baras A. Genetic and pharmacologic inactivation of ANGPTL3 and cardiovascular disease. N Engl J Med 2017;377:211–221.
83. Stitziel NO, Khera AV, Wang X, Bierhals AJ, Vourakis AC, Sperry AE, Natarajan P, Klarin D, Emdin CA, Zekavat SM, Nomura A, Erdmann J, Schunkert H, Samani NJ, Kraus WE, Shah SH, Yu B, Boerwinkle E, Rader DJ, Gupta N, Frossard PM, Rasheed A, Danesh J, Lander ES, Gabriel S, Saleheen D, Musunuru K, Kathiresan S. ANGPTL3 deficiency and protection against coronary artery disease. J Am Coll Cardiol 2017;69:2054–2063.
84. Graham MJ, Lee RG, Brandt TA, Tai LJ, Fu W, Peralta R, Yu R, Hurh E, Paz E, McEvoy BW, Baker BF, Pham NC, Digenio A, Hughes SG, Geary RS, Witztum JL, Crooke RM, Tsimikas S. Cardiovascular and metabolic effects of ANGPTL3 antisense oligonucleotides. N Engl J Med 2017;377:222–232.
85. Khera AV, Won H-H, Peloso GM, O’Dushlaine C, Liu D, Stitziel NO, Natarajan P, Nomura A, Emdin CA, Gupta N, Borecki IB, Asselta R, Duga S, Merlini PA, Correa A, Kessler T, Wilson JG, Bown MJ, Hall AS, Braund PS, Carey DJ, Murray MF, Kirchner HL, Leader JB, Lavage DR, Manus JN, Hartzel DN, Samani NJ, Schunkert H, Marrugat J, Elosua R, McPherson R, Farrall M, Watkins H, Lander ES, Rader DJ, Danesh J, Ardissino D, Gabriel S, Willer C, Abecasis GR, Saleheen D, Dewey FE, Kathiresan S. Association of rare and common variation in the lipoprotein lipase gene with coronary artery disease. JAMA 2017;317:937–946.
86. Nordestgaard BG, Abildgaard S, Wittrup HH, Steffensen R, Jensen G, Tybjaerg-Hansen A. Heterozygous lipoprotein lipase deficiency: frequency in the general population, effect on plasma lipid levels, and risk of ischemic heart disease. Circulation 1997;96:1737–1744.
87. Beigneux AP, Miyashita K, Ploug M, Blom DJ, Ai M, Linton MF, Khovidhunkit W, Dufour R, Garg A, McMahon MA, Pullinger CR, Sandoval NP, Hu X, Allan CM, Larsson M, Machida T, Murakami M, Reue K, Tontonoz P, Goldberg IJ, Moulin P, Charriere S, Fong LG, Nakajima K, Young SG. Autoantibodies against GPIHBP1 as a cause of hypertriglyceridemia. N Engl J Med 2017;376:1647–1658.
88. Afshar M, Luk K, Do R, Dufresne L, Owens DS, Harris TB, Peloso GM, Kerr KF, Wong Q, Smith AV, Budoff MJ, Rotter JI, Cupples LA, Rich SS, Engert JC, Gudnason V, O’Donnell CJ, Post WS, Thanassoulis G. Association of triglyceride-related genetic variants with mitral annular calcification. J Am Coll Cardiol 2017;69:2941–2948.
89. Nordestgaard BG. A test in context: lipid profile, fasting versus nonfasting. J Am Coll Cardiol 2017;70:1637–1646.
90. Lincoff AM, Nicholls SJ, Riesmeyer JS, Barter PJ, Brewer HB, Fox KAA, Gibson CM, Granger C, Menon V, Montalescot G, Rader D, Tall AR, McErlean E, Wolski K, Ruotolo G, Vangerow B, Weerakkody G, Goodman SG, Conde D, McGuire DK, Nicolau JC, Leiva-Pons JL, Pesant Y, Li W, Kandath D, Kouz S, Tahirkheli N, Mason D, Nissen SE. Evacetrapib and cardiovascular outcomes in high-risk vascular disease. N Engl J Med 2017;376:1933–1942.
91. Schwartz GG, Olsson AG, Abt M, Ballantyne CM, Barter PJ, Brumm J, Chaitman BR, Holme IM, Kallend D, Leiter LA, Leitersdorf E, McMurray JJ, Mundl H, Nicholls SJ, Shah PK, Tardif JC, Wright RS. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med 2012;367:2089–2099.
92. Tardif JC, Rhainds D, Rheaume E, Dube MP. CETP: pharmacogenomics-based response to the CETP inhibitor dalcetrapib. Arterioscler Thromb Vasc Biol 2017; 37: 396–400.
93. Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, Lopez-Sendon J, Mosca L, Tardif JC, Waters DD, Shear CL, Revkin JH, Buhr KA, Fisher MR, Tall AR, Brewer B. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med 2007; 357: 2109–2122.
94. Ference BA, Kastelein JJP, Ginsberg HN, Chapman MJ, Nicholls SJ, Ray KK, Packard CJ, Laufs U, Brook RD, Oliver-Williams C, Butterworth AS, Danesh J, Smith GD, Catapano AL, Sabatine MS. Association of genetic variants related to CETP inhibitors and statins with lipoprotein levels and cardiovascular risk. JAMA 2017; 318: 947–956.
95. Speer T, Rohrer L, Blyszczuk P, Shroff R, Kuschnerus K, Krankel N, Kania G, Zewinger S, Akhmedov A, Shi Y, Martin T, Perisa D, Winnik S, Muller MF, Sester U, Wernicke G, Jung A, Gutteck U, Eriksson U, Geisel J, Deanfield J, von EA, Luscher TF, Fliser D, Bahlmann FH, Landmesser U. Abnormal high-density lipoprotein induces endothelial dysfunction via activation of Toll-like receptor-2. Immunity 2013; 38: 754–768.
96. Zewinger S, Kleber ME, Rohrer L, Lehmann M, Triem S, Jennings RT, Petrakis I, Dressel A, Lepper PM, Scharnagl H, Ritsch A, Thorand B, Heier M, Meisinger C, de Las Heras GT, Koenig W, Wagenpfeil S, Schwedhelm E, Boger RH, Laufs U, von EA, Landmesser U, Luscher TF, Fliser D, Marz W, Meinitzer A, Speer T. Symmetric dimethylarginine, high-density lipoproteins and cardiovascular disease. Eur Heart J 2017; 38: 1597–1607.
97. Madsen CM, Varbo A, Nordestgaard BG. Extreme high high-density lipoprotein cholesterol is paradoxically associated with high mortality in men and women: two prospective cohort studies. Eur Heart J 2017; 38: 2478–2486.
98. Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005; 352: 1685–1695.
99. Coussens LM, Werb Z. Inflammation and cancer. Nature 2002; 420: 860–867.
100. Ridker PM, MacFadyen JG, Thuren T, Everett BM, Libby P, Glynn RJ, Ridker P, Lorenzatti A, Krum H, Varigos J, Siostrzonek P, Sinnaeve P, Fonseca F, Nicolau J, Gotcheva N, Genest J, Yong H, Urina-Triana M, Milicic D, Cifkova R, Vettus R, Koenig W, Anker SD, Manolis AJ, Wyss F, Forster T, Sigurdsson A, Pais P, Fucili A, Ogawa H, Shimokawa H, Veze I, Petrauskiene B, Salvador L, Kastelein J, Cornel JH, Klemsdal TO, Medina F, Budaj A, Vida-Simiti L, Kobalava Z, Otasevic P, Pella D, Lainscak M, Seung K-B, Commerford P, Dellborg M, Donath M, Hwang J-J, Kultursay H, Flather M, Ballantyne C, Bilazarian S, Chang W, East C, Everett B, Forgosh L, Glynn R, Harris B, Libby P, Ligueros M, Thuren T, Bohula E, Charmarthi B, Cheng S, Chou S, Danik J, McMahon G, Maron B, Ning MMing, Olenchock B, Pande R, Perlstein T, Pradhan A, Rost N, Singhal A, Taqueti V, Wei N, Burris H, Cioffi A, Dalseg AM, Ghosh N, Gralow J, Mayer T, Rugo H, Fowler V, Limaye AP, Cosgrove S, Levine D, Lopes R, Scott J, Thuren T, Ligueros M, Hilkert R, Tamesby G, Mickel C, Manning B, Woelcke J, Tan M, Manfreda S, Ponce T, Kam J, Saini R, Banker K, Salko T, Nandy P, Tawfik R, O’Neil G, Manne S, Jirvankar P, Lal S, Nema D, Jose J, Collins R, Bailey K, Blumenthal R, Colhoun H, Gersh B, Glynn RJ. Effect of interleukin-1beta inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet 2017; 390: 1833–1842.
101. Ridker PM, MacFadyen JG, Everett BM, Libby P, Thuren T, Glynn RJ; on behalf of the CANTOS Trial Group. Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: a secondary analysis from the CANTOS randomised controlled trial. Lancet 2017; pii: S0140-6736(17)32814-3. doi: https://doi.org/10.1016/S0140-6736(17)32814-3.
102. Baylis RA, Gomez D, Mallat Z, Pasterkamp G, Owens GK. The CANTOS trial: one important step for clinical cardiology but a giant leap for vascular biology. Arterioscler Thromb Vasc Biol 2017; 37: e174–e177.
103. Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, Darius H, Lewis BS, Ophuis TO, Jukema JW, De Ferrari GM, Ruzyllo W, De LP, Im K, Bohula EA, Reist C, Wiviott SD, Tershakovec AM, Musliner TA, Braunwald E, Califf RM. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015; 372: 2387–2397.
104. Ginsberg HN, Elam MB, Lovato LC, Crouse JRIII, Leiter LA, Linz P, Friedewald WT, Buse JB, Gerstein HC, Probstfield J, Grimm RH, Ismail-Beigi F, Bigger JT, Goff DC Jr, Cushman WC, Simons-Morton DG, Byington RP. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med 2010; 362: 1563–1574.
105. Wu JH, Foote C, Blomster J, Toyama T, Perkovic V, Sundstrom J, Neal B. Effects of sodium-glucose cotransporter-2 inhibitors on cardiovascular events, death, and major safety outcomes in adults with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol 2016; 4: 411–419.
106. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE. Empagliflozin, cardiovascular outcomes, and mortality in Type 2 diabetes. N Engl J Med 2015; 373: 2117–2128.
107. Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von EM, Mattheus M, Johansen OE, Woerle HJ, Broedl UC, Zinman B. Empagliflozin and progression of kidney disease in Type 2 diabetes. N Engl J Med 2016; 375: 323–334.
108. Neal B, Perkovic V, Mahaffey KW, de ZD, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR. Canagliflozin and cardiovascular and renal events in Type 2 diabetes. N Engl J Med 2017; 377: 644–657.
109. Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM, Stockner M, Zinman B, Bergenstal RM, Buse JB. Liraglutide and cardiovascular outcomes in Type 2 diabetes. N Engl J Med 2016; 375: 311–322.
110. Mann JFE, Orsted DD, Brown-Frandsen K, Marso SP, Poulter NR, Rasmussen S, Tornoe K, Zinman B, Buse JB. Liraglutide and renal outcomes in Type 2 diabetes. N Engl J Med 2017; 377: 839–848.
111. Kosiborod M, Cavender MA, Fu AZ, Wilding JP, Khunti K, Holl RW, Norhammar A, Birkeland KI, Jorgensen ME, Thuresson M, Arya N, Bodegard J, Hammar N, Fenici P. Lower risk of heart failure and death in patients initiated on sodium-glucose cotransporter-2 inhibitors versus other glucose-lowering drugs: the CVD-REAL study (comparative effectiveness of cardiovascular outcomes in new users of sodium-glucose cotransporter-2 inhibitors). Circulation 2017; 136: 249–259.
112. Birkeland KI, Jorgensen ME, Carstensen B, Persson F, Gulseth HL, Thuresson M, Fenici P, Nathanson D, Nystrom T, Eriksson JW, Bodegard J, Norhammar A. Cardiovascular mortality and morbidity in patients with type 2 diabetes following initiation of sodium-glucose co-transporter-2 inhibitors versus other glucose-lowering drugs (CVD-REAL Nordic): a multinational observational analysis. Lancet Diabetes Endocrinol 2017; 5: 709–717.
113. Kernan WN, Viscoli CM, Furie KL, Young LH, Inzucchi SE, Gorman M, Guarino PD, Lovejoy AM, Peduzzi PN, Conwit R, Brass LM, Schwartz GG, Adams HP Jr, Berger L, Carolei A, Clark W, Coull B, Ford GA, Kleindorfer D, O’Leary JR, Parsons MW, Ringleb P, Sen S, Spence JD, Tanne D, Wang D, Winder TR. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med 2016; 374: 1321–1331.
114. Vaccaro O, Masulli M, Nicolucci A, Bonora E, Del PS, Maggioni AP, Rivellese AA, Squatrito S, Giorda CB, Sesti G, Mocarelli P, Lucisano G, Sacco M, Signorini S, Cappellini F, Perriello G, Babini AC, Lapolla A, Gregori G, Giordano C, Corsi L, Buzzetti R, Clemente G, Di CG, Iannarelli R, Cordera R, La MO, Zamboni C, Scaranna C, Boemi M, Iovine C, Lauro D, Leotta S, Dall’Aglio E, Cannarsa E, Tonutti L, Pugliese G, Bossi AC, Anichini R, Dotta F, Di BA, Citro G, Antenucci D, Ricci L, Giorgino F, Santini C, Gnasso A, De CS, Zavaroni D, Vedovato M, Consoli A, Calabrese M, di BP, Fornengo P, Riccardi G. Effects on the incidence of cardiovascular events of the addition of pioglitazone versus sulfonylureas in patients with type 2 diabetes inadequately controlled with metformin (TOSCA.IT): a randomised, multicentre trial. Lancet Diabetes Endocrinol 2017; 5: 887–897.
115. Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygina Y, Buse JB, Chan JC, Choi J, Gustavson SM, Iqbal N, Maggioni AP, Marso SP, Ohman P, Pagidipati NJ, Poulter N, Ramachandran A, Zinman B, Hernandez AF. Effects of once-weekly exenatide on cardiovascular outcomes in Type 2 diabetes. N Engl J Med 2017; 377: 1228–1239.
116. Marso SP, McGuire DK, Zinman B, Poulter NR, Emerson SS, Pieber TR, Pratley RE, Haahr PM, Lange M, Brown-Frandsen K, Moses A, Skibsted S, Kvist K, Buse JB. Efficacy and safety of degludec versus glargine in Type 2 diabetes. N Engl J Med 2017; 377: 723–732.
117. Johansen MY, MacDonald CS, Hansen KB, Karstoft K, Christensen R, Pedersen M, Hansen LS, Zacho M, Wedell-Neergaard AS, Nielsen ST, Iepsen UW, Langberg H, Vaag AA, Pedersen BK, Ried-Larsen M. Effect of an intensive lifestyle intervention on glycemic control in patients with Type 2 diabetes. A Randomized Clinical Trial. JAMA 2017; 318: 637–646.
118. Nordestgaard BG, Palmer TM, Benn M, Zacho J, Tybjærg-Hansen A, Davey Smith G, Timpson NJ, Minelli C. The effect of elevated body mass index on ischemic heart disease risk: causal estimates from a Mendelian randomisation approach. PLoS Med 2012; 9: e1001212.
119. Afzal S, Brondum-Jacobsen P, Bojesen SE, Nordestgaard BG. Vitamin D concentration, obesity, and risk of diabetes: a Mendelian randomisation study. Lancet Diabetes Endocrinol 2014; 2: 298–306.
120. Emdin CA, Khera AV, Natarajan P, Klarin D, Zekavat SM, Hsiao AJ, Kathiresan S. Genetic association of waist-to-hip ratio with cardiometabolic traits, Type 2 diabetes, and coronary heart disease. JAMA 2017; 317: 626–634.
121. Dale CE, Fatemifar G, Palmer TM, White J, Prieto-Merino D, Zabaneh D, Engmann JEL, Shah T, Wong A, Warren HR, McLachlan S, Trompet S, Moldovan M, Morris RW, Sofat R, Kumari M, Hypponen E, Jefferis BJ, Gaunt TR, Ben-Shlomo Y, Zhou A, Gentry-Maharaj A, Ryan A, Mutsert R, Noordam R, Caulfield MJ, Jukema JW, Worrall BB, Munroe PB, Menon U, Power C, Kuh D, Lawlor DA, Humphries SE, Mook-Kanamori DO, Sattar N, Kivimaki M, Price JF, Davey SG, Dudbridge F, Hingorani AD, Holmes MV, Casas JP. Causal associations of adiposity and body fat distribution with coronary heart disease, stroke subtypes, and Type 2 diabetes mellitus: a Mendelian randomization analysis. Circulation 2017; 135: 2373–2388.
122. Lee SS, Ae KK, Kim D, Lim YM, Yang PS, Yi JE, Kim M, Kwon K, Bum PW, Joung B, Park J. Clinical implication of an impaired fasting glucose and prehypertension related to new onset atrial fibrillation in a healthy Asian population without underlying disease: a nationwide cohort study in Korea. Eur Heart J 2017; 38: 2599–2607.
123. Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Bohm M. Renal sympathetic denervation in patients with treatment-resistant hypertension (the symplicity HTN-2 trial): a randomised controlled trial. Lancet 2010; 376: 1903–1909.
124. Bhatt DL, Kandzari DE, O’Neill WW, D’Agostino R, Flack JM, Katzen BT, Leon MB, Liu M, Mauri L, Negoita M, Cohen SA, Oparil S, Rocha-Singh K, Townsend RR, Bakris GL. A controlled trial of renal denervation for resistant hypertension. N Engl J Med 2014; 370: 1393–1401.
125. Townsend RR, Mahfoud F, Kandzari DE, Kario K, Pocock S, Weber MA, Ewen S, Tsioufis K, Tousoulis D, Sharp ASP, Watkinson AF, Schmieder RE, Schmid A, Choi JW, East C, Walton A, Hopper I, Cohen DL, Wilensky R, Lee DP, Ma A, Devireddy CM, Lea JP, Lurz PC, Fengler K, Davies J, Chapman N, Cohen SA, DeBruin V, Fahy M, Jones DE, Rothman M, Bohm M. Catheter-based renal denervation in patients with uncontrolled hypertension in the absence of antihypertensive medications (SPYRAL HTN-OFF MED): a randomised, sham-controlled, proof-of-concept trial. Lancet 2017; 390: 2160–2170.
126. Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC Jr, Fine LJ, Cutler JA, Cushman WC, Cheung AK, Ambrosius WT. A Randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373: 2103–2116.
127. Berlowitz DR, Foy CG, Kazis LE, Bolin LP, Conroy MB, Fitzpatrick P, Gure TR, Kimmel PL, Kirchner K, Morisky DE, Newman J, Olney C, Oparil S, Pajewski NM, Powell J, Ramsey T, Simmons DL, Snyder J, Supiano MA, Weiner DE, Whittle J. Effect of intensive blood-pressure treatment on patient-reported outcomes. N Engl J Med 2017; 377: 733–744.
128. Bavishi C, Bangalore S, Messerli FH. Outcomes of intensive blood pressure lowering in older hypertensive patients. J Am Coll Cardiol 2017; 69: 486–493.
129. Niiranen TJ, McCabe EL, Larson MG, Henglin M, Lakdawala NK, Vasan RS, Cheng S. Risk for hypertension crosses generations in the community: a multi-generational cohort study. Eur Heart J 2017; 38: 2300–2308.
130. Anand SS, Bosch J, Eikelboom JW, Connolly SJ, Diaz R, Widimsky P, Aboyans V, Alings M, Kakkar AK, Keltai K, Maggioni AP, Lewis BS, Störk S, Zhu J, Lopez-Jaramillo P, O’Donnell M, Commerford PJ, Vinereanu D, Pogosova N, Ryden L, Fox KAA, Bhatt DL, Misselwitz F, Varigos JD, Vanassche T, Avezum AA, Chen E, Branch K, Leong DP, Bangdiwala SI, Hart RG, Yusuf S; COMPASS Investigators. Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: an international, randomised, double-blind, placebo-controlled trial. Lancet 2017; pii: S0140-6736(17)32409-1. doi: https://doi.org/10.1016/S0140-6736(17)32409-1.
131. Ohman EM, Roe MT, Steg PG, James SK, Povsic TJ, White J, Rockhold F, Plotnikov A, Mundl H, Strony J, Sun X, Husted S, Tendera M, Montalescot G, Bahit MC, Ardissino D, Bueno H, Claeys MJ, Nicolau JC, Cornel JH, Goto S, Kiss RG, Guray U, Park DW, Bode C, Welsh RC, Gibson CM. Clinically significant bleeding with low-dose rivaroxaban versus aspirin, in addition to P2Y12 inhibition, in acute coronary syndromes (GEMINI-ACS-1): a double-blind, multicentre, randomised trial. Lancet 2017; 389: 1799–1808.
132. Godier A, Dincq AS, Martin AC, Radu A, Leblanc I, Antona M, Vasse M, Golmard JL, Mullier F, Gouin-Thibault I. Predictors of pre-procedural concentrations of direct oral anticoagulants: a prospective multicentre study. Eur Heart J 2017; 38: 2431–2439.
133. Lindholt JS, Sogaard R. Population screening and intervention for vascular disease in Danish men (VIVA): a randomised controlled trial. Lancet 2017; 390: 2256–2265.
134. Karthikesalingam A, Vidal-Diez A, Holt PJ, Loftus IM, Schermerhorn ML, Soden PA, Landon BE, Thompson MM. Thresholds for abdominal aortic aneurysm repair in England and the United States. N Engl J Med 2016; 375: 2051–2059.
135. Geisel MH, Bauer M, Hennig F, Hoffmann B, Lehmann N, Mohlenkamp S, Kroger K, Kara K, Muller T, Moebus S, Erbel R, Scherag A, Jockel KH, Mahabadi AA. Comparison of coronary artery calcification, carotid intima-media thickness and ankle-brachial index for predicting 10-year incident cardiovascular events in the general population. Eur Heart J 2017; 38: 1815–1822.
136. Nilsson J. Atherosclerotic plaque vulnerability in the statin era. Eur Heart J 2017; 38: 1638–1644.
137. van der Valk FM, Kuijk C, Verweij SL, Stiekema LCA, Kaiser Y, Zeerleder S, Nahrendorf M, Voermans C, Stroes ESG. Increased haematopoietic activity in patients with atherosclerosis. Eur Heart J 2017; 38: 425–432.
138. Kunutsor SK, Seidu S, Khunti K. Statins and secondary prevention of venous thromboembolism: pooled analysis of published observational cohort studies. Eur Heart J 2017; 38: 1608–1612.
139. Glynn RJ, Danielson E, Fonseca FA, Genest J, Gotto AM Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Ridker PM. A randomized trial of rosuvastatin in the prevention of venous thromboembolism. N Engl J Med 2009; 360: 1851–1861.
140. Weitz JI, Lensing AWA, Prins MH, Bauersachs R, Beyer-Westendorf J, Bounameaux H, Brighton TA, Cohen AT, Davidson BL, Decousus H, Freitas MCS, Holberg G, Kakkar AK, Haskell L, van BB, Pap AF, Berkowitz SD, Verhamme P, Wells PS, Prandoni P. Rivaroxaban or aspirin for extended treatment of venous thromboembolism. N Engl J Med 2017; 376: 1211–1222.
141. Stroes ES, Thompson PD, Corsini A, Vladutiu GD, Raal FJ, Ray KK, Roden M, Stein E, Tokgozoglu L, Nordestgaard BG, Bruckert E, De BG, Krauss RM, Laufs U, Santos RD, Hegele RA, Hovingh GK, Leiter LA, Mach F, Marz W, Newman CB, Wiklund O, Jacobson TA, Catapano AL, Chapman MJ, Ginsberg HN. Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society Consensus Panel statement on assessment, aetiology and management. Eur Heart J 2015; 36: 1012–1022.
142. Vonbank A, Agewall S, Kjeldsen KP, Lewis BS, Torp-Pedersen C, Ceconi C, Funck-Brentano C, Kaski JC, Niessner A, Tamargo J, Walther T, Wassmann S, Rosano G, Schmidt H, Saely CH, Drexel H. Comprehensive efforts to increase adherence to statin therapy. Eur Heart J 2017; 38: 2473–2479.
143. Nielsen SF, Nordestgaard BG. Negative statin-related news stories decrease statin persistence and increase myocardial infarction and cardiovascular mortality: a nationwide prospective cohort study. Eur Heart J 2016; 37: 908–916.
144. Matthews A, Herrett E, Gasparrini A, Van ST, Goldacre B, Smeeth L, Bhaskaran K. Impact of statin related media coverage on use of statins: interrupted time series analysis with UK primary care data. BMJ 2016; 353: i3283.
145. Zhang H, Plutzky J, Shubina M, Turchin A. Continued statin prescriptions after adverse reactions and patient outcomes: a cohort study. Ann Intern Med 2017; 167: 221–227.
146. Serban MC, Colantonio LD, Manthripragada AD, Monda KL, Bittner VA, Banach M, Chen L, Huang L, Dent R, Kent ST, Muntner P, Rosenson RS. Statin intolerance and risk of coronary heart events and all-cause mortality following myocardial infarction. J Am Coll Cardiol 2017; 69: 1386–1395.
147. Gupta A, Thompson D, Whitehouse A, Collier T, Dahlof B, Poulter N, Collins R, Sever P. Adverse events associated with unblinded, but not with blinded, statin therapy in the anglo-scandinavian cardiac outcomes trial-lipid-lowering arm (ASCOT-LLA): a randomised double-blind placebo-controlled trial and its non-randomised non-blind extension phase. Lancet 2017; 389: 2473–2481.
148. Rosenson RS, Baker S, Banach M, Borow KM, Braun LT, Bruckert E, Brunham LR, Catapano AL, Elam MB, Mancini GBJ, Moriarty PM, Morris PB, Muntner P, Ray KK, Stroes ES, Taylor BA, Taylor VH, Watts GF, Thompson PD. Optimizing cholesterol treatment in patients with muscle complaints. J Am Coll Cardiol 2017; 70: 1290–1301.
149. NICE Guidance. Cardiovascular disease: risk assessment and reduction, including lipid modification. https://www.nice.org.uk/guidance/cg181 (June 17 2017).
150. Anderson TJ, Gregoire J, Pearson GJ, Barry AR, Couture P, Dawes M, Francis GA, Genest J Jr, Grover S, Gupta M, Hegele RA, Lau DC, Leiter LA, Lonn E, Mancini GB, McPherson R, Ngui D, Poirier P, Sievenpiper JL, Stone JA, Thanassoulis G, Ward R. 2016 Canadian cardiovascular society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol 2016; 32: 1263–1282.
151. Leung AA, Nerenberg K, Daskalopoulou SS, McBrien K, Zarnke KB, Dasgupta K, Cloutier L, Gelfer M, Lamarre-Cliche M, Milot A, Bolli P, Tremblay G, McLean D, Tobe SW, Ruzicka M, Burns KD, Vallee M, Prasad GV, Lebel M, Feldman RD, Selby P, Pipe A, Schiffrin EL, McFarlane PA, Oh P, Hegele RA, Khara M, Wilson TW, Penner SB, Burgess E, Herman RJ, Bacon SL, Rabkin SW, Gilbert RE, Campbell TS, Grover S, Honos G, Lindsay P, Hill MD, Coutts SB, Gubitz G, Campbell NR, Moe GW, Howlett JG, Boulanger JM, Prebtani A, Larochelle P, Leiter LA, Jones C, Ogilvie RI, Woo V, Kaczorowski J, Trudeau L, Petrella RJ, Hiremath S, Drouin D, Lavoie KL, Hamet P, Fodor G, Gregoire JC, Lewanczuk R, Dresser GK, Sharma M, Reid D, Lear SA, Moullec G, Gupta M, Magee LA, Logan AG, Harris KC, Dionne J, Fournier A, Benoit G, Feber J, Poirier L, Padwal RS, Rabi DM. Hypertension Canada’s 2016 Canadian hypertension education program guidelines for blood pressure measurement, diagnosis, assessment of risk, prevention, and treatment of hypertension. Can J Cardiol 2016; 32: 569–588.
152. Bibbins-Domingo K, Grossman DC, Curry SJ, Davidson KW, Epling JW Jr, Garcia FA, Gillman MW, Kemper AR, Krist AH, Kurth AE, Landefeld CS, LeFevre ML, Mangione CM, Phillips WR, Owens DK, Phipps MG, Pignone MP. Statin use for the primary prevention of cardiovascular disease in adults: US preventive services task force recommendation statement. JAMA 2016; 316: 1997–2007.
153. Jellinger PS, Handelsman Y, Rosenblit PD, Bloomgarden ZT, Fonseca VA, Garber AJ, Grunberger G, Guerin CK, Bell DSH, Mechanick JI, Pessah-Pollack R, Wyne K, Smith D, Brinton EA, Fazio S, Davidson M. American Association of Clinical Endocrinologists and American College of Endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract 2017; 23: 1–87.
154. Lloyd-Jones DM, Morris PB, Ballantyne CM, Birtcher KK, Daly DD Jr, DePalma SM, Minissian MB, Orringer CE, Smith SC Jr. 2017 focused update of the 2016 ACC expert consensus decision pathway on the role of non-statin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American College of Cardiology task force on expert consensus decision pathways. J Am Coll Cardiol 2017; 70: 1785–1822.
155. Mortensen MB, Nordestgaard BG, Afzal S, Falk E. ACC/AHA guidelines superior to ESC/EAS guidelines for primary prevention with statins in non-diabetic Europeans: the Copenhagen general population study. Eur Heart J 2017; 38: 586–594.
156. Mortensen MB, Falk E. Limitations of the SCORE-guided European guidelines on cardiovascular disease prevention. Eur Heart J 2017; 38: 2259–2263.
157. Ray KK, Kastelein JJ. Time to change the SCORE? Eur Heart J 2017; 38: 595–597.
158. Leening MJG, Cook NR, Ridker PM. Should we reconsider the role of age in treatment allocation for primary prevention of cardiovascular disease? Eur Heart J 2017; 38: 1542–1547.
159. Jackson R, Kerr A, Wells S. ‘Should we reconsider the role of age in treatment allocation for primary prevention of cardiovascular disease?’ No, but we can improve risk communication metrics. Eur Heart J 2017; 38: 1548–1552.
160. DeFilippis AP, Young R, McEvoy JW, Michos ED, Sandfort V, Kronmal RA, McClelland RL, Blaha MJ. Risk score overestimation: the impact of individual cardiovascular risk factors and preventive therapies on the performance of the American Heart Association-American College of Cardiology-Atherosclerotic Cardiovascular Disease risk score in a modern multi-ethnic cohort. Eur Heart J 2017; 38: 598–608.
161. Hippisley-Cox J, Coupland C, Brindle P. Development and validation of QRISK3 risk prediction algorithms to estimate future risk of cardiovascular disease: prospective cohort study. BMJ 2017; 357: j2099.
162. Nordestgaard BG, Langsted A, Mora S, Kolovou G, Baum H, Bruckert E, Watts GF, Sypniewska G, Wiklund O, Boren J, Chapman MJ, Cobbaert C, Descamps OS, von EA, Kamstrup PR, Pulkki K, Kronenberg F, Remaley AT, Rifai N, Ros E, Langlois M. Fasting is not routinely required for determination of a lipid profile: clinical and laboratory implications including flagging at desirable concentration cut-points-a joint consensus statement from the European Atherosclerosis Society and European Federation of Clinical Chemistry and Laboratory Medicine. Eur Heart J 2016; 37: 1944–1958.
163. Scartezini M, Ferreira CEDS, Izar MCO, Bertoluci M, Vencio S, Campana GA, Sumita NM, Barcelos LF, Faludi AA, Santos RD, Malachias MVB, Aquino JL, Galoro CAO, Sabino C, Gurgel MHC, Turatti LAA, Hohl A, Martinez TLDR. Positioning about the flexibility of fasting for lipid profiling. Arq Bras Cardiol 2017; 108: 195–197.
164. Miller M, Stone NJ, Ballantyne C, Bittner V, Criqui MH, Ginsberg HN, Goldberg AC, Howard WJ, Jacobson MS, Kris-Etherton PM, Lennie TA, Levi M, Mazzone T, Pennathur S. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation 2011; 123: 2292–2333.
165. 2Downs JR, O’Malley PG. Management of dyslipidemia for cardiovascular disease risk reduction: synopsis of the 2014 U.S. Department of Veterans Affairs and U.S. Department of Defense clinical practice guideline. Ann Intern Med 2015; 163: 291–297.
166. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison HC, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol 2017; pii: S0735-1097(17)41519-1. doi: https://doi.org/10.1016/j.jacc.2017.11.006.