The year in cardiology: coronary interventions
█ Current opinion
Authors:
Andreas Baumbach1,2*, Christos V. Bourantas1,2,3, Patrick W. Serruys4,5, William Wijns5
1Department of Cardiology, Barts Heart Centre, Barts Health NHS Trust, West Smithfield, London, EC1A 7BE, UK;
2Centre for Cardiovascular Medicine and Devices, William
Harvey Research Institute, Queen Mary University of London, London, UK;
3Institute of Cardiovascular
Sciences, University College London, London, UK;
4International Centre for Circulatory Health, Imperial College London, London, UK; and
5The Lambe Institute for Translational Medicine and Curam, Saolta University
Healthcare Group, National University of Ireland Galway, Galway, Ireland
Received 3 December 2019; revised 16 December 2019; editorial decision 17 December 2019;
accepted 24 December 2019; online publish-ahead-of-print 6 January 2020
Preamble
Percutaneous coronary intervention (PCI) research focuses on the optimization of treatment strategies, the development of novel equipment and pharmacotherapies for improved results, and on risk stratification and identification of high-risk patients that will benefit from emerging therapies targeting atherosclerotic evolution. Over the last year, important clinical studies have been reported that examined the efficacy of different treatment strategies and stent platforms in patients with obstructive coronary artery disease (CAD) and guidelines have been published to provide recommendations about the management of these patients. The aim of this article is to summarize the findings of the pivotal studies published in 2019 and discuss their impact on clinical practice.
ISSUE: CARDIOLOGIA HUNGARICA | 2020 | VOLUME 50, ISSUE 4
Revascularization in patients with cardiac arrest or acute coronary syndromes
Coronary Angiography after Cardiac Arrest (COACT) is a landmark study that changed the management of patients admitted with a cardiac arrest who had successful resuscitation and no ST elevation myocardial infarction (STEMI) (1). In this prospective multicentre trial, 552 patients admitted with an out of hospital cardiac arrest with an initial shockable rhythm who did not have an obvious non-cardiac cause of arrest were randomized to immediate coronary angiography and if needed coronary revascularization or delayed coronary angiography after neurological recovery. An acute thrombotic occlusion was noted only in 3.4% of the patients in the immediate angiography and in 7.6% of the patient in the delayed angiography group. Survival rate at discharge (65.2% vs. 68.7%) and at 90-day follow-up (64.5% vs. 67.2%) was not different between randomization groups. In addition, there was no difference for the incidence of the composite endpoint survival with good cerebral performance or mild or moderate disability (62.9% vs. 64.4%). These findings contradict previous observational studies that penalized a delayed invasive assessment of the coronary artery anatomy and justify both approaches in this setting.
Conversely, the Complete vs. Culprit-Only Revascularization Strategies to Treat Multivessel Disease after Early PCI for STEMI (COMPLETE) study confirmed the value of an aggressive revascularization strategy in patients admitted with a STEMI (2). In this study, 4041 patients who had multivessel CAD were randomized in a 1:1 ratio to complete revascularization vs. culprit-lesion-only PCI. At 3-year follow-up, the incidence of the composite endpoint cardiovascular death or myocardial infarction (MI) was lower in patients undergoing complete revascularization as compared to the patients that had PCI only in the culprit vessel (7.8% vs. 10.5%; P = 0.004); of note, the benefit of complete revascularization was similar in patients who had an in-hospital second procedure compared to a procedure following readmission within 45 days post-discharge; however, this comparison was not randomized, as the choice for timing of the second procedure was left to operator’s discretion. The prognostic value of complete revascularization in patients with non-STEMI has not been fully investigated yet.
Chronic coronary syndromes
Revascularization vs. medical therapyDespite the robust evidence supporting the prognostic implications of complete revascularization in patients admitted with a STEMI, studies examining the value of PCI in improving outcomes in patients with a chronic coronary syndrome show mixed results. A retrospective analysis including 16,029 patients who had positron emission computed tomography myocardial perfusion imaging demonstrated that an early surgical or percutaneous revascularization was associated with improved prognosis in patients with an ischaemic burden >5–10% (3). These findings, however, were not confirmed in a post hoc analysis of the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial that included 1379 patients who had stress perfusion imaging and quantitative coronary angiography (4). At 7.9 years of follow-up, the extent of CAD – defined by the number of the diseased vessels – and not the severity of ischaemia was a predictor of survival. Percutaneous coronary intervention in this cohort did not improve outcomes over optimal medical therapy; more importantly, there was no interaction between the extent of ischaemia or CAD and the treatment strategy (i.e. conservative vs. PCI).
In line with these findings, the International Study Of Comparative Health Effectiveness With Medical And Invasive Approaches (ISCHEMIA study) that included 5179 patients, with moderate or severe ischaemia in non-invasive imaging, who were randomized to optimal medical therapy or optimal medical therapy plus PCI demonstrated no differences in outcomes between groups at 3.3 years of follow-up for the composite endpoint of cardiovascular death, MI, admission for unstable angina, heart failure symptoms, or resuscitated cardiac arrest (15.5% vs. 13.8%, P = 0.34) (5). In this study, PCI was associated with an improvement in the quality of life, a reduction in the angina symptoms and a lower incidence of spontaneous MI [hazard ratio (HR) 0.67, 95% confidence interval (CI) 0.53–0.83; P < 0.01]. An important limitation of the ISCHEMIA study is the high (28%) crossover rate from the conservative to the invasive arm which may have affected the reported results; the as-treated analysis has not been reported yet.
The association between the presence of viable myocardium, surgical revascularization, and clinical outcomes was recently evaluated by a post hoc analysis of the Surgical Treatment for Ischaemic Heart Failure (STICH) study (6). This analysis that included 601 patients who had a left ventricular ejection fraction ≤35% and viability assessment, failed to demonstrate an impact of the presence or absence of myocardial viability on the survival benefit noted in patients undergoing surgical revascularization at 10.4-year follow-up. The REVascularisation for Ischaemic VEntricular Dysfunction (REVIVED) study (NCT01920048) is currently examining the safety and efficacy of PCI in improving prognosis in patients with heart failure.
Patient and lesion subset
Left main and three-vessel disease
The optimal revascularization strategy in patients with advanced CAD [i.e. three-vessel disease or left main stem (LMS) disease] and in diabetic patients has been discussed in the 2018 European Society of Cardiology (ESC) Guidelines on myocardial revascularization: surgical revascularization is currently the recommended treatment strategy in diabetic patients with multivessel CAD, while PCI has a IIB indication in patients with a SYNTAX score ≤22 and is not recommended in patients with SYNTAX score >22 (7).
These recommendations are in line with the findings of the Future Revascularization Evaluation in Patients with Diabetes Mellitus: Optimal Management of Multivessel Disease (FREEDOM) Follow-On study that included 1900 diabetic patients with multivessel disease that were randomized to surgical or percutaneous revascularization and reported a higher mortality rate at 8 years of follow-up in the PCI arm compared to the surgical revascularization group (24.3% vs. 18.3%, P = 0.010) (8). Conversely, the Synergy between PCI with Taxus and Cardiac Surgery (SYNTAX) Extended Survival study that included 1689 patients with LMS or three-vessel disease did not demonstrate differences in the all-cause mortality between patients allocated to PCI and those treated surgically at 10 years of follow-up (27% vs. 24%, P = 0.092). There was, however, a treatment effect by subgroup interaction according to the presence or absence of three-vessel disease; mortality was increased in the PCI group compared to the coronary artery bypass graft (CABG) arm (HR 1.41, 95% CI: 1.10–1.80), while there was no differences between the two groups in patients with LMS disease (HR 0.90, 95% CI: 0.68–1.20); conversely, there was no difference in outcomes for the two treatment strategies in diabetic and non-diabetic patients (P-for interaction 0.660) (9). A limitation of both studies is the fact that the patients in the PCI arm were treated with a 1st generation drug-eluting stent (DES) that is not currently used in contemporary practice, and the fact that they both reported only all-cause mortality instead of patient-orientated cardiovascular endpoints.
The Evaluation of XIENCE vs. Coronary Artery Bypass Surgery for Effectiveness of Left Main Revascularization (EXCEL) study overcame these limitations; in this study, 1905 patients with LMS disease and SYNTAX score ≤32 were randomized to PCI with a 2nd generation DES or CABG (10). In the PCI arm, intravascular ultrasound (IVUS) imaging was used in 77.2% of the cases (11). At 5-year follow-up, there were no differences between groups for the combined endpoint of all-cause death, MI, or stroke (22.0% in the PCI arm vs. 19.2% in the CABG group; P = 0.13). The event rate at 30-day follow-up was lower in the PCI arm (4.9% vs. 8.0%), there was no difference between groups for the period 30 days to 1 year (4.1% vs. 3.8%), while for the period 1–5 years of follow-up a higher event rate was reported in patients undergoing PCI (15.1% vs. 9.7%). Patients randomized to CABG were more likely to suffer a cerebrovascular event (5.2% vs. 3.3%), while those treated with PCI had increased all-cause mortality (13.0% vs. 9.9%) and more often ischaemia driven revascularization (16.9% vs. 10.0%). Similarly to what it has been reported in the SYNTAX study, there was no difference in the outcomes between the two treatment strategies in diabetic and non-diabetic patients at 3- and 5-year follow-up (10, 12).
Percutaneous coronary intervention of bifurcation stenoses
In 2019, the 3-year follow-up data of the DKCRUSH V study were published; similar to what has been reported at 1-year follow-up, double kiss-crush technique was associated with a lower incidence of target lesion revascularization (TLR, 5.0% vs. 10.3%, P=0.029) target vessel MI (1.7% vs. 5.8%, P=0.017), and definite or probable stent thrombosis (0.4% vs. 4.1%, P=0.006) compared to provisional T-stenting (13). Double kiss-crush technique, however, is a challenging procedure and requires skills and expertise; therefore, considering that the findings of the DKCRUSH V study may not be reproduced by centres with less experienced operators, the recently published 14th consensus document from the European Bifurcation Club advocates the use of provisional T-stenting technique for the treatment of bifurcations lesions and proposes a two stent strategy only in lesions with a complex anatomy, when access to the side branch is challenging, or when there is ostial disease in the side branches extending >5 mm form the carina and/or increased calcification (14). In the case of a two stent strategy, the European Bifurcation Club recommends the use of culotte or TAP technique and when the crush technique is considered it proposes the use of the double kiss-crush.
Treatment of chronic total occlusions
In 2019, the EuroCTO Club published a consensus document that summarizes the current evidence, discusses the indications for chronic total occlusion (CTO) revascularization, presents the advances in CTO equipment, and provides recommendations about training in CTO PCI (15). In line with the ESC guidelines on myocardial revascularization and taking into account the findings of randomized controlled studies, the EuroCTO Club recommends CTO recanalization in the presence of symptoms despite optimal medical therapy; in asymptomatic patients, ischaemic burden assessment is recommended and CTO revascularization is advised if there is evidence of increased ischaemic burden (≥10% of the left ventricular mass). These recommendation are in line with the findings of the recently reported Drug-Eluting Stent Implantation vs. Optimal Medical Treatment in Patients With Chronic Total Occlusion (DECISION-CTO) trial (16). In this study, 815 patients with a CTO were randomized in 1:1 ratio to complete revascularization or to the treatment of the obstructive non-CTO lesions whenever these were present. Only one-fourth of the patients included in the two groups had a single-vessel disease. At 4-year follow-up, there was no difference between the two groups for the combined endpoint of death, MI, stroke, or revascularization (22.4% vs. 22.3%, P=0.86) or patients’ quality of life. These findings indicate that in case of multivessel disease revascularization of the non-CTO lesion and re-evaluation of the extent of ischaemia and patient symptoms should be considered before advocating recanalization of a CTO. Limitations of the study – the largest of its kind – included the high crossover rate (19.6%) from the non-CTO PCI group to the CTO-PCI group within the first days from randomization as well the fact that it was underpowered for the primary endpoint as patient recruitment was early terminated because of a slow enrolment rate.
Small vessel and in-stent restenosis
Percutaneous coronary intervention in small vessels has been associated with a higher incidence of major adverse cardiovascular events (MACE) and TLR due to in-stent restenosis. In 2019, a pre-specified sub-analysis of the Biodegradable Polymer and Durable Polymer Drug-eluting Stents in an All Comers Population (BIO-RESORT) study was published that compared outcomes following PCI in small vessels (<2.5 mm) using ultrathin-strut cobalt chromium biodegradable polymer sirolimus-eluting stents (strut thickness 71 μm) or very thin-strut platinum chromium biodegradable polymer everolimus-eluting stents (strut thickness 78 μm) or previous-generation thin strut cobalt-chromium durable polymer zotarolimus-eluting stents (strut thickness 102 μm). A higher incidence of TLR was noted in the thicker strut zotarolimus-eluting stent than the ultrathin-strut sirolimus-eluting stent group (5.3% vs. 2.1%, P=0.006), while there was no difference in the TLR rate between the everolimus and zotarolimus-eluting stent groups (4.0% vs. 5.1%, P=0.31) (17). These findings convincingly highlight the prognostic implications of strut thickness in small vessels in the DES era and are in line with previous studies reporting outcomes in bare-metal stents (18).
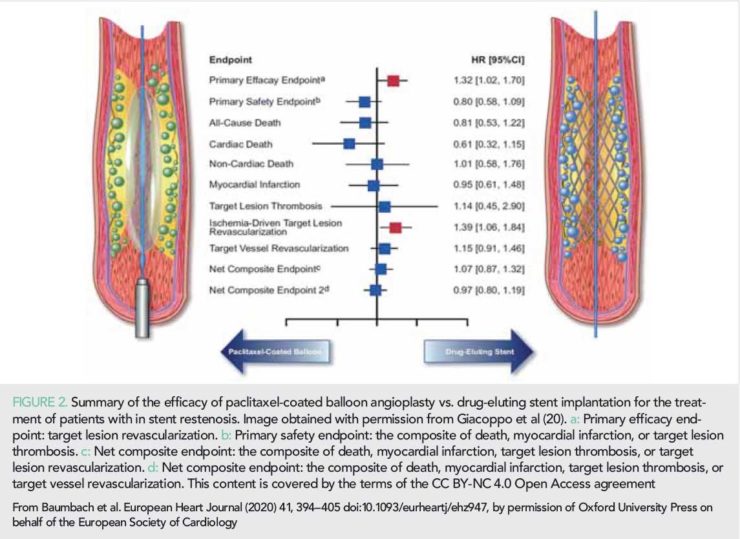
In-stent restenosis represents the most common cause of stent failure; its treatment is challenging and is associated with poor prognosis and a high TLR rate (19). The two most effective treatment strategies today are drug-coated balloon angioplasty or DES implantation. In 2019, the Difference in Anti-restenotic Effectiveness of Drug-eluting stent and drug-coated balloon AngiopLasty for the occUrrence of coronary in-Stent restenosis (DAEDALUS) patient-level meta-analysis was published that included 1976 patients treated with a paclitaxel-coated balloon or a DES (20). At 3-year follow-up, paclitaxel-coated balloon angioplasty was associated with a higher incidence of TLR comparing to DES implantation (HR 1.32, 95% CI: 1.02–1.70; P=0.035); however, there was no difference between groups for the combined endpoint of death, MI, or target lesion thrombosis (Figure 2).
Existing and emerging interventional devices
Drug-eluting stents and bioresorbable scaffolds
The ESC Guidelines on myocardial revascularization recommends the use of 2nd generation DES in daily clinical practice (7). The extended follow-up of the Comparison of Biolimus Eluted From an Erodible Stent Coating With Bare Metal Stents in Acute ST-Elevation Myocardial Infarction (COMFORTABLE-AMI) study and the nested intravascular imaging analysis published this year has provided further evidence about the superiority of DES over bare-metal stents in patients admitted with a STEMI. At 5-year follow-up, Biolimus stent implantation was associated with a lower incidence of target vessel MI (2.2% vs. 5.0, P=0.02) and ischaemia driven TLR (4.4% vs. 10.4%, P<0.001) than treatment with a bare-metal stent (21).
The BIOSTEMI study also focused on the treatment of patients with STEMI and randomized 1300 subjects to ultrathin cobalt chromium sirolimus-eluting stent vs. durable polymer everolimus-eluting stent implantation. At 12-month follow-up, treatment with ultrathin sirolimus-eluting stents was associated with a lower incidence of target lesion failure (TLF) than everolimus-eluting stents (4% vs. 6%; rate ratio: 0.59, 95% Bayesian credibility interval: 0.37–0.94; posterior probability of superiority 0.986) (22). Conversely, the TALENT study that compared outcomes in all-comer patients randomized to ultrathin cobalt chromium sirolimus-eluting stent and durable polymer everolimus-eluting stent failed to show a difference for the incidence of the composite endpoint of cardiac death, target-vessel MI, or clinically indicated TLR between groups (4.9% vs. 5.3%, Pfor non-inferiority <0.0001) (23).
Bioresorbable scaffolds have been introduced to overcome the limitations of DES and improve long-term outcomes. However, the increased event rate reported in these devices at short- and intermediate-term follow-up raised concerns about their safety and today are not recommended for routine clinical use. A recent meta-analysis of randomized studies comparing the Absorb bioresorbable vascular scaffold (BVS) and the everolimus-eluting stent showed a higher incidence of TLF in the Absorb BVS at 5-year follow-up (14.9% vs. 11.6%, P = 0.030) that was attributed to a higher incidence of target vessel MI and ischaemia driven TLR (24). Landmark analysis demonstrated a higher event rate in the Absorb BVS group for the period 0–3 years of follow-up; however, for the period 3–5 years of follow-up, the incidence of cardiac death, target vessel MI, ischaemia driven TLR, and device thrombosis was similar between groups in patients who had not experienced an event in the first 3 years. These findings for the first time provide unique insights about the timing of the events in bioresobable scaffolds and indicate a low event rate at long term after their full resorption.
Adjunctive interventional devices
Intravascular lithotripsy (IVL) has emerged over the last years as an effective alternative for the treatment of calcified lesions that are associated with an increased risk of complications and worse prognosis (25). It involves the advancement of a catheter with a balloon on its tip that contains multiple emitters which generate sonic pressure waves that selectively fracture vascular calcium without affecting the integrity of the fibroelastic tissue of the plaque (26). The Shockwave Coronary Rx Lithoplasty Study (DISRUPT CAD) was the first study that systematically examined the safety and efficacy of IVL in 60 patients with heavily calcified lesions and length ≤32 mm; the procedure was successful in all the lesions resulting in an acute gain of 1.7 mm and a post-procedural percent diameter stenosis of 12.2%. The overall MACE rate at 6 months of follow-up was 8.3%; three peri-procedural MI and two cardiac deaths were reported (27). Similar were the findings of the DISRUPT CAD II study that included 120 patients; in that study, the in-hospital MACE rate was 5.8% (7 non-Q wave MI), while at 30-day follow-up, the MACE rate was 7.6%. Optical coherence tomography (OCT) imaging was performed in 48 patients before and in 47 after stenting and demonstrated that IVL caused 3.4 ± 2.6 fractures per lesion resulting in an acute gain of 4.79 ± 2.45 mm2 and an excellent stent expansion of 102.8 ± 30.6% (28). Recently, Wilson et al. (29) showed that IVL therapy is associated with ventricular ectopics and asynchronous pacing. In this study, no malignant arrhythmias were reported; the ongoing DISRUPT CAD III study is expected to provide further evidence about the safety and efficacy of IVL in the treatment of calcified lesions (NCT03595176).
Adjunctive pharmacotherapy
The type and the duration of antiplatelet therapy in patients undergoing PCI is an area of intensive research. The Ticagrelor with Aspirin or Alone in High-Risk Patients after Coronary Intervention (TWILIGHT) study was designed to examine the optimal duration of dual antiplatelet therapy (DAPT) following PCI in high bleeding risk patients (30). The study randomized 7119 patients to DAPT therapy for 3 months and then treatment with ticagrelor monotherapy or DAPT for 12 months. Short duration DAPT was associated with a lower incidence of bleeding [rate of Bleeding Academic Research Consortium (BARC) type 2, 3, and 5 bleeding: 4.0% in the short duration DAPT group vs. 7.1% in the group receiving DAPT for 12 months, P < 0.001], while there was no difference between groups in the incidence of the composite endpoint death, MI, or stroke.
Conversely, a post hoc analysis of the Global Leaders study including 4570 patients undergoing complex PCI demonstrated that the experimental regimen of aspirin for 1 month and ticagrelor for 24 months was associated with a lower incidence of the primary endpoint death, MI at 2 years of follow-up compared to conventional DAPT for 12 months and then aspirin monotherapy (3.51% vs. 5.43%; P=0.002). Of note, there was no difference between groups in the risk of bleeding (incidence of BARC type 3 or 5 bleeding: 2.45% vs. 2.54%; P=0.834). These findings were confirmed by a patient-level analysis of eight randomized control trials including 14,963 patients which demonstrated that in low bleeding risk patients (PREdicting bleeding Complications in patients undergoing stent Implantation and SubsequEnt Dual AntiPlatelet Therapy score <25) prolonged DAPT therapy was associated with a lower incidence of ischaemic events especially in patients undergoing complex PCI. Conversely, long-term DAPT in high bleeding risk patients did not reduce the risk of ischaemic events and increased the risk of bleeding (31).
Patients suffering from atrial fibrillation undergoing PCI are at increased risk of bleeding as they receive a combination of antiplatelet and anticoagulation therapy. The optimal treatment of these patients has been extensively investigated by several large scale randomized control studies over the last years. The AUGUSTUS trial published this year was a multicentre randomized study with a 2×2 factorial design that randomized 4614 patients with atrial fibrillation undergoing PCI to treatment with a P2Y12 inhibitor, and apixaban or vitamin K antagonist, and to aspirin or placebo for 6 months (32). The recruited patients received standard of care antithrombotic therapy the first days post-PCI as randomization to study groups was performed 6 (interquartile range 3–10) days post-intervention. The incidence of major or clinically relevant non-major bleeding was higher in patients receiving vitamin K antagonist than those treated with apixaban (14.7% vs. 10.5%, P<0.001) and in those treated with aspirin than those receiving placebo (16.1% vs. 9.0%, P<0.001). Patients on apixaban had a lower incidence of death or hospitalization than the vitamin K antagonist group (23.5% vs. 27.4%, P = 0.002) and a similar incidence of ischaemic events. Conversely, aspirin did not have an effect to these endpoints.
Similar were the findings of the ENTRUST-AF PCI study which investigated in 1506 patients with atrial fibrillation undergoing PCI the safety and efficacy of the combination of a P2Y12 inhibitor plus edoxaban against the combination DAPT plus vitamin K antagonist (33). The recruited patients were randomized to the two study groups ≈45 h post-PCI. There was no difference between groups in the incidence of major bleeding-clinically relevant non-significant bleeding or the incidence of the composite endpoint of cardiovascular death, stroke, systemic embolic events, MI, and definite stent thrombosis at 12 months of follow-up. A meta-analysis of randomized controlled trials investigating the safety and efficacy of dual vs. triple antithrombotic therapy in patients with atrial fibrillation undergoing PCI, published this year, confirmed the above findings demonstrating a lower incidence of bleeding (13.4% vs. 20.8%; P<0.0001) but a higher risk of stent thrombosis (1% vs. 0.6%; P=0.040) in patients receiving dual therapy (34).
Invasive diagnostic tools
Coronary physiology
Recent studies have shown that the fractional flow reserve (FFR) and the resting indices including the instantaneous wave free ratio (iwFR) have a value not only in guiding revascularization but also in assessing the final results post-PCI and predicting prognosis (35, 36). There are however occasional discordances between hyperaemic FFR and resting indices. Several studies this year attempted to examine the physiological characteristics of lesions with discordant FFR and iwFR and identify lesion types and subgroup of patients where FFR should be preferred to iwFR and vice versa (37, 38). A recent sub-analysis of the Functional Lesion Assessment of Intermediate Stenosis to Guide Revascularization (DEFINE-FLAIR) study comparing outcomes in patients with a lesion in the left anterior descending coronary artery deferred from revascularization based on the FFR or iwFR estimations showed a lower event rate in the iwFR group at 1-year follow-up that was attributed to a lower incidence of unplanned revascularizations (2.22% vs. 4.99%, P=0.03) (39). Conversely, a post hoc analysis of the same study in diabetic patients showed no differences in outcomes between the FFR and iwFR groups (7.2% vs. 10.0%; P=0.30); however, the incidence of non-fatal MI was higher in the iwFR group (4.7% vs. 1.9%; P=0.05) with a significant interaction for the presence of diabetes (P = 0.04) (40).
In parallel with the introduction of the resting indices for the assessment of the functional severity of intermediate lesions, efforts have been made to design computerized-based methodologies that are able to post-process coronary angiography or invasive imaging data to derive FFR. In 2019, two new solutions have been presented for computational-derived FFR: the first relies on three-dimensional quantitative coronary angiography to derive vessel geometry and estimate the pressure drop across a lesion, while the second on the processing of OCT imaging data; the latter enables combined morphological and physiological assessment of atherosclerotic lesions and of the procedural results post-PCI (41, 42). Preliminary validation of these solutions showed promising results; however, further evaluation of their efficacy in a large number of patients is required before their broad application in the clinical arena.
Intravascular imaging
Cumulative evidence has highlighted the value of IVUS in guiding PCI. A meta-analysis of randomized controlled trials published this year including 4724 patients underscored the prognostic benefit of IVUS guidance, demonstrating a lower incidence in MACE (5.4% vs. 9.0%; P< 0.001), cardiac death (0.6% vs. 1.2%, P=0.03), TLR (3.1% vs. 5.2%, P=0.001), and definite/probable stent thrombosis (0.5% vs. 1.1%, P=0.02) rates in the IVUS-guided compared to the angiography-guided group (43). In line with the above findings, the 5-year follow-up analysis of the Impact of Intravascular Ultrasound Guidance on Outcomes of XIENCE PRIME Stents in Long Lesions (IVUS-XPL) study that included 1400 patients with long lesions ≥28 mm randomized to IVUS- and angiography-guided PCI, reported a lower incidence of MACE (5.6% vs. 10.7%, P=0.001) in the IVUS-guided group attributed to a lower incidence of TLR (4.8% vs. 8.4%, P=0.007). A landmark analysis for the follow-up period 1–5 years indicated that IVUS guidance was associated with clinical benefit at long-term follow-up (HR 0.53, 95% CI 0.29–0.95; P=0.031) (44). These findings highlight the prognostic implications of IVUS in guiding revascularization and support its routine use to optimize procedural results and improve the short- and long-term outcomes post-PCI.
Fractional flow reserve is currently recommended to guide revascularization in patients with a chronic coronary syndrome and intermediate lesions. The FORZA study examined the value of OCT in deferring PCI; the study included 350 patients with intermediate lesions who were randomized to OCT- and FFR-guided PCI (45). Revascularization in the OCT group was performed based on area stenosis and minimum lumen area cut-off values, while in the FFR group PCI was performed if the FFR was ≤0.80. OCT and FFR were repeated in the two groups and used to optimize stent deployment. At 13 months of follow-up, OCT-guided PCI was associated with a higher incidence of revascularization and increased cost while there was no difference in the incidence of MACE – defined as the composite endpoint of all-cause death, MI, target vessel revascularization – between the FFR- and OCT-guided groups (8.0% vs. 3.4%, P=0.064). For the primary endpoint of the study, i.e. the incidence of MACE and significant angina at 13 months of follow-up, OCT-guided PCI was marginally superior to FFR-guidance (14.8% vs. 8.0%, P=0.048). The FROZA study is the first that compared in a randomized fashion intravascular imaging vs. physiology guided PCI revealing limitations of both approaches in guiding revascularization (i.e. increased procedural cost and number of vessels treated in the OCT-guided group and a higher incidence of MACE and angina symptoms in the FFR-guided group). Combined physiology and imaging-guided revascularization is likely to overcome the limitations of both modalities and optimize procedural results and the clinical outcomes of patients with obstructive CAD.
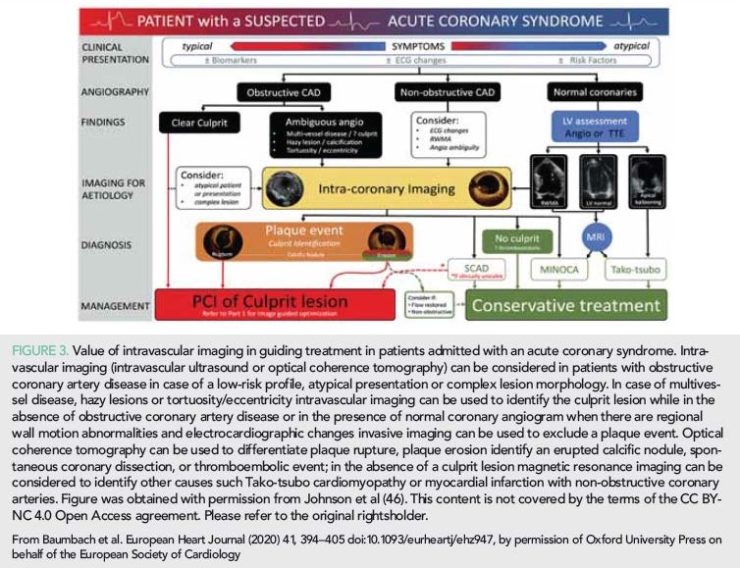
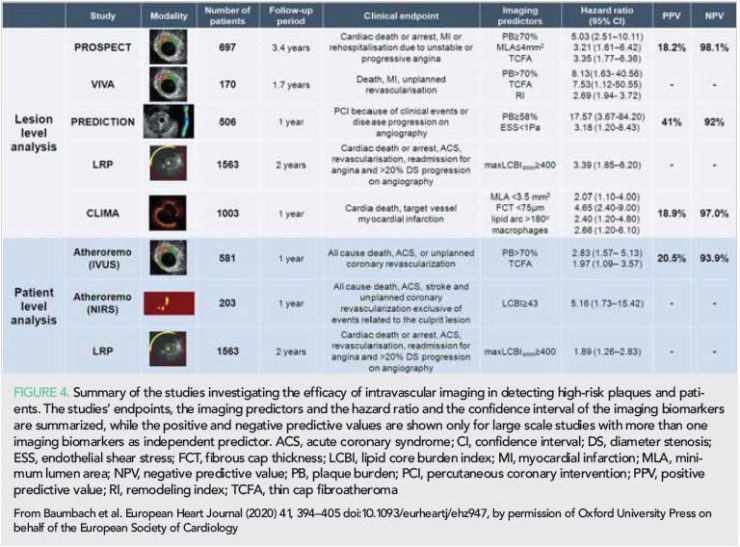
In 2019, the European Association of Percutaneous Cardiovascular Interventions published an expert consensus document about the value of intravascular imaging in guiding treatment in ACS and in ambiguous coronary angiography findings (46). This report highlights the value of intravascular imaging and in particular of OCT in identifying the culprit lesion when this cannot be detected by coronary angiography and in tailoring therapy in patients admitted with an ACS (Figure 3). It also underscores the value of intravascular imaging in assessing ambiguous coronary angiographic findings, in detecting embolic events and intramural haematomas, in assessing lesions caused by an external compression of the vessel by other organs and it summarizes the evidence that supports its role in identifying vulnerable plaques and high-risk patients (Figure 4).
Non-invasive imaging
Non-invasive functional imaging has an established role in the diagnosis of obstructive CAD in symptomatic patients (47). In the Myocardial Perfusion CMR vs. Angiography and FFR to Guide the Management of Patients with Stable Coronary Artery Disease (MR-INFORM) study, non-invasive imaging and in particular cardiac magnetic resonance (CMR) imaging was found to be not only useful for the diagnosis of CAD but also for guiding revascularization (48). In this study, 918 patients were randomized to CMR- or FFR-guided revascularization. CMR-guided PCI was associated with a lower incidence of coronary angiography and PCI (35.7% vs. 45.0%, P=0.005). At 1-year follow-up, there was no difference between groups for the primary endpoint of all-cause mortality, MI, or target vessel revascularization (3.6% vs. 3.7%, P=0.91). This report is among the few that compared the role of non-invasive imaging vs. invasive guidance for PCI. A limitation of this study is the fact that the event rate was lower than the 10% event rate assumed in the power calculation and thus it may have been underpowered in detecting differences in outcomes between the two study groups.
Similar were the findings of the Complete Revascularization or Stress Echocardiography in Patients With Multivessel Disease and ST-Segment Elevation Acute Myocardial Infarction (CROSS-AMI) study that compared angiography vs. stress echocardiography-guided revascularization in patients admitted with a STEMI that had non-culprit lesions with a diameter stenosis >50% on quantitative coronary angiography (49). The study was prematurely stopped after enrolling 77% of the patients because of a slow recruitment (n=306). The authors reported a higher incidence of non-culprit lesion revascularization in the angiography group (88% vs. 22%). At 1-year follow-up, there were no differences between groups for the primary endpoint of cardiac death, MI, coronary revascularization, or re-admission because of heart failure (14% vs. 14%, P=0.85). A limitation of the CROSS-AMI study was the fact that it was underpowered to assess differences between groups. Therefore, further research is needed to examine the value of non-invasive imaging in guiding revascularization in patients with an ACS.
Vulnerable plaque and patient detection
The event rate of patients undergoing revascularization and especially of those admitted with an ACS is high- at short-term follow-up (50). The identification of high-risk patients has recently attracted attention as novel pharmacotherapies have been introduced that appear able to modify atherosclerotic plaque and inhibit disease progression. However, these new therapies have significant limitations as they are associated with increased cost or side effects. Accurate risk stratification and identification of high-risk individuals is expected to allow a personalized therapy and aggressive treatment of these patients with novel medications that appear to improve outcomes in vulnerable populations (51).
Large scale prospective intravascular imaging studies of coronary atherosclerosis have highlighted the value of IVUS in detecting vulnerable plaques that are likely to progress and cause events and in stratifying more accurately cardiovascular risk. In 2019, the Lipid-Rich Plaque (LRP) and the CLIMA studies were reported which for the first time assessed the efficacy of near-infrared spectroscopy (NIRS)-IVUS and of OCT in detecting vulnerable plaques (52, 53). The LRP registry included 1563 patients with suspected CAD that had coronary angiography and possible ad hoc PCI. NIRS-IVUS imaging was performed in the non-culprit vessels in at least two major coronary arteries with length >50 mm. At 2-year follow-up, patients with increased lipid burden (4 mm lipid core burden index, maxLCBI4mm >400) had a higher incidence of non-culprit MACE than those with lipid-free plaques (13% vs. 6%, P < 0.0001). Patient-level (adjusted HR 1.89, 95% CI: 1.26–2.83; P=0.0021) and lesion-level (adjusted HR 3.39, 95% CI: 1.85–6.20; P<0.0001) analysis demonstrated that maxLCBI4mm>400 was independent predictor of MACE at 2-year follow-up. The LRP study provided evidence for the prognostic implications of plaque composition but it failed to investigate the synergetic value of NIRS and IVUS in predicting events as IVUS analysis was not complete but restricted to the 4 mm segment with the maxLCBI.
The CLIMA study was a prospective multicentre registry that investigated the prognostic implications of OCT-derived plaque characteristic in 1003 patients who had coronary angiography for clinical purposes and OCT imaging of the untreated proximal left anterior descending coronary artery (53). In this study, a minimum lumen area <3.5 mm2, a lipid arc >180°, a fibrous cap thickness <75 µm, and the presence of macrophages accumulations were independent predictors of the combined endpoint cardiac death and target segment MI. Patients having lesions with all the above plaque features had a higher event rate than the other patients (18.9% vs. 3.0%, P<0.001).
Advances in coronary imaging
Summarizing the results of these studies and taking into consideration the findings of previous reports it appears that plaque characteristics provides useful prognostic information at a lesion and patient level; but they have a limited accuracy in predicting events. Over the last years, several methodologies have been introduced to enhance the efficacy of the existing modalities in assessing plaque characteristics and an effort has been made to develop hybrid-multimodality intravascular imaging catheters that will allow a complete assessment of plaque morphology and biology. In 2019, the first in man application of the combined IVUS-OCT catheter has been presented (54). In addition, this year the first in man application of a polarization sensitive OCT imaging system was presented; this modality is expected to enable better plaque characterization and more detailed evaluation of its components (55). Finally, two reports have recently examined the efficacy of attenuation compensation technique, a post-processing methodology that appears able to enhance OCT imaging depth and enable more accurate evaluation of plaque burden in heavily diseased segments (56, 57). These reports highlighted the potential of this approach in assessing plaque area in heavily diseased native vessels but also demonstrated significant limitations of this technique, because of imaging artefacts, in stented segments.
Cumulative evidence has highlighted the implications of the local haemodynamic forces on atherosclerotic disease progression and destabilization. In 2019, an analysis of the Integrated Biomarkers Imaging Study 4 (IBIS-4) has shown that the shear stress distribution estimated using computational fluid dynamic analysis adds value in predicting atherosclerotic disease progression and changes in plaque morphology, while a meta-analysis of the Providing Regional Observations to Study Predictors of Events in the Coronary Tree (PROSPECT) study has shown that estimation of plaque stress by processing virtual histology-IVUS images enables more accurate identification of lesions that will cause events in future (58, 59). Acknowledging the importance of the local haemodynamic forces on atherosclerotic disease progression in native and stented segments expert recommendations have been recently published in a consensus document which describes the existing methodologies and their value for research and possibly clinical practice in the future (60).
Conclusions
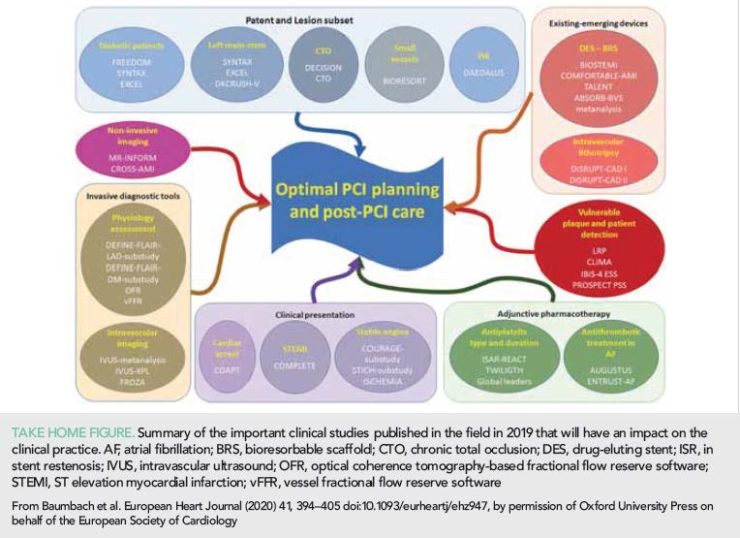
Published research in 2019 examining the efficacy of different treatment strategies, of emerging or existing devices and of the value of coronary physiology or intravascular imaging in PCI planning has enriched our understanding and modified the treatment of patients with obstructive CAD (Take home figure). Patients suffering from a STEMI should be treated aggressively aiming for complete revascularization. Conversely, an initially conservative management in patients with an out of hospital cardiac arrest without clinical evidence of ongoing acute ischaemia seems to be equally effective as an early invasive approach. Robust evidence highlights the short- and long-term efficacy of DES, while advances in coronary physiology and the development of image-based methodologies for the computation of FFR are expected to broaden its use in guiding revascularization. Cumulative data underscore the prognostic benefit of intravascular imaging in guiding PCI and in assessing lesion pathology, while advances in intravascular imaging and computational modelling are anticipated to allow better prediction of vulnerable lesions and of patients at risk that will benefit from emerging therapies targeting plaque evolution. These developments are expected to improve procedural results and long-term outcomes in patients with CAD through personalized pharmaco-invasive strategies.
Conflict of interest
A.B. has received institutional research support from Abbott Vascular and honoraria from Astra Zeneca, Sinomed, Microport, Abbott Vascular, Cardinal Health, KSH. C.V.B. has no conflict to declare. P.W.S. reports personal fees from Biosensors, Micel Technologies, Sinomedical Sciences Technology, Philips/Volcano, Xeltis, and HeartFlow, outside the submitted work. W.W. has received speaker fees from Biotronik, MicroPort and Terumo; institutional research grants from Abbott Vascular, MiCell, MicroPort and Terumo; Medical Advisor for Rede Optimus Research and co-founder of Argonauts Partners, an innovation facilitator.
References
1. Lemkes JS, Janssens GN, van der Hoeven NW, Jewbali LSD, Dubois EA, Meuwissen M, Rijpstra TA, Bosker HA, Blans MJ, Bleeker GB, Baak R, Vlachojannis GJ, Eikemans BJW, van der Harst P, van der Horst ICC, Voskuil M, van der Heijden JJ, Beishuizen A, Stoel M, Camaro C, van der Hoeven H, Henriques JP, Vlaar APJ, Vink MA, van den Bogaard B, Heestermans T, de Ruijter W, Delnoij TSR, Crijns H, Jessurun GAJ, Oemrawsingh PV, Gosselink MTM, Plomp K, Magro M, Elbers PWG, van de Ven PM, Oudemans-van Straaten HM, van Royen N. Coronary angiography after cardiac arrest without ST-segment elevation. N Engl J Med 2019; 380: 1397–1407.
2. Mehta SR, Wood DA, Storey RF, Mehran R, Bainey KR, Nguyen H, Meeks B, Di Pasquale G, Lopez-Sendon J, Faxon DP, Mauri L, Rao SV, Feldman L, Steg PG, Avezum A, Sheth T, Pinilla-Echeverri N, Moreno R, Campo G, Wrigley B, Kedev S, Sutton A, Oliver R, Rodes-Cabau J, Stankovic G, Welsh R, Lavi S, Cantor WJ, Wang J, Nakamya J, Bangdiwala SI, Cairns JA; COMPLETE Trial Steering Committee and Investigators. Complete revascularization with multivessel PCI for myocardial infarction. N Engl J Med 2019; 381: 1411–1421.
3. Patel KK, Spertus JA, Chan PS, Sperry BW, Thompson RC, Al Badarin F, Kennedy KF, Case JA, Courter S, Saeed IM, McGhie AI, Bateman TM. Extent of myocardial ischemia on positron emission tomography and survival benefit with early revascularization. J Am Coll Cardiol 2019; 74: 1645–1654.
4. Weintraub WS, Hartigan PM, Mancini GBJ, Teo KK, Maron DJ, Spertus JA, Chaitman BR, Shaw LJ, Berman D, Boden WE. Effect of coronary anatomy and myocardial ischemia on long-term survival in patients with stable ischemic heart disease. Circ Cardiovasc Qual Outcomes 2019; 12: e005079.
5. Hochman JS. International Study Of Comparative Health Effectiveness With Medical And Invasive Approaches (ISCHEMIA): primary report of clinical outcomes. American Heart Association Scientific Sessions 2019, Philadelphia 2019. Nov 16–18.
6. Panza JA, Ellis AM, Al-Khalidi HR, Holly TA, Berman DS, Oh JK, Pohost GM, Sopko G, Chrzanowski L, Mark DB, Kukulski T, Favaloro LE, Maurer G, Farsky PS, Tan RS, Asch FM, Velazquez EJ, Rouleau JL, Lee KL, Bonow RO. Myocardial viability and long-term outcomes in ischemic cardiomyopathy. N Engl J Med 2019; 381: 739–748.
7. Neumann F-J, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet J-P, Falk V, Head SJ, Jüni P, Kastrati A, Koller A, Kristensen SD, Niebauer J, Richter DJ, Seferović PM, Sibbing D, Stefanini GG, Windecker S, Yadav R, Zembala MO; ESC Scientific Document Group. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 2019; 40: 87–165.
8. Farkouh ME, Domanski M, Dangas GD, Godoy LC, Mack MJ, Siami FS, Hamza TH, Shah B, Stefanini GG, Sidhu MS, Tanguay J-F, Ramanathan K, Sharma SK, French J, Hueb W, Cohen DJ, Fuster V, Sharma SK, Zazif TN, Thai H, Shah B, Ramanathan K, Tanguay J-F, Ramanathan K, Burton JR, Schampaert E, Escobedo J, Dubois-Rande J-L, Macaya C, Carrie D, Richardt G, Roguin A, Lotan C, Kornowski R, Presbitero P, Hueb W, Sousa JE, Velásquez JG, Rodriguez A, Devlin G, French JK, Kaul U; FREEDOM Follow-On Study Investigators. Long-term survival following multivessel revascularization in patients with diabetes: the FREEDOM Follow-On Study. J Am Coll Cardiol 2019; 73: 629–638.
9. Thuijs DJFM, Kappetein AP, Serruys PW, Mohr F-W, Morice M-C, Mack MJ, Holmes DR, Curzen N, Davierwala P, Noack T, Milojevic M, Dawkins KD, da Costa BR, Jüni P, Head SJ; SYNTAX Extended Survival Investigators. Percutaneous coronary intervention versus coronary artery bypass grafting in patients with three-vessel or left main coronary artery disease: 10-year follow-up of the multicentre randomised controlled SYNTAX trial. Lancet 2019; 394: 1325–1334.
10. Stone GW, Kappetein AP, Sabik JF, Pocock SJ, Morice MC, Puskas J, Kandzari DE, Karmpaliotis D, Brown WM3rd, Lembo NJ, Banning A, Merkely B, Horkay F, Boonstra PW, van Boven AJ, Ungi I, Bogats G, Mansour S, Noiseux N, Sabate M, Pomar J, Hickey M, Gershlick A, Buszman PE, Bochenek A, Schampaert E, Page P, Modolo R, Gregson J, Simonton CA, Mehran R, Kosmidou I, Genereux P, Crowley A, Dressler O, Serruys PW; EXCEL Trial Investigators. Five-year outcomes after PCI or CABG for left main coronary disease. N Engl J Med 2019; 381: 1820–1830.
11. Stone GW, Sabik JF, Serruys PW, Simonton CA, Genereux P, Puskas J, Kandzari DE, Morice MC, Lembo N, Brown WM3rd, Taggart DP, Banning A, Merkely B, Horkay F, Boonstra PW, van Boven AJ, Ungi I, Bogats G, Mansour S, Noiseux N, Sabate M, Pomar J, Hickey M, Gershlick A, Buszman P, Bochenek A, Schampaert E, Page P, Dressler O, Kosmidou I, Mehran R, Pocock SJ, Kappetein AP; EXCEL Trial Investigators. Everolimus-eluting stents or bypass surgery for left main coronary artery disease. N Engl J Med 2016; 375: 2223–2235.
12. Milojevic M, Serruys PW, Sabik JF3rd, Kandzari DE, Schampaert E, van Boven AJ, Horkay F, Ungi I, Mansour S, Banning AP, Taggart DP, Sabate M, Gershlick AH, Bochenek A, Pomar J, Lembo NJ, Noiseux N, Puskas JD, Crowley A, Kosmidou I, Mehran R, Ben-Yehuda O, Genereux P, Pocock SJ, Simonton CA, Stone GW, Kappetein AP. Bypass surgery or stenting for left main coronary artery disease in patients with diabetes. J Am Coll Cardiol 2019; 73: 1616–1628.
13. Chen X, Li X, Zhang JJ, Han Y, Kan J, Chen L, Qiu C, Santoso T, Paiboon C, Kwan TW, Sheiban I, Leon MB, Stone GW, Chen SL; DKCRUSH-V Investigators. 3-year outcomes of the DKCRUSH-V trial comparing DK crush with provisional stenting for left main bifurcation lesions. JACC Cardiovasc Interv 2019; 12: 1927–1937.
14. Banning AP, Lassen JF, Burzotta F, Lefevre T, Darremont O, Hildick-Smith D, Louvard Y, Stankovic G. Percutaneous coronary intervention for obstructive bifurcation lesions: the 14th consensus document from the European Bifurcation Club. EuroIntervention 2019; 15: 90–98.
15. Galassi AR, Werner GS, Boukhris M, Azzalini L, Mashayekhi K, Carlino M, Avran A, Konstantinidis NV, Grancini L, Bryniarski L, Garbo R, Bozinovic N, Gershlick AH, Rathore S, Di Mario C, Louvard Y, Reifart N, Sianos G. Percutaneous recanalisation of chronic total occlusions: 2019 consensus document from the EuroCTO Club. EuroIntervention 2019; 15: 198–208.
16. Lee SW, Lee PH, Ahn JM, Park DW, Yun SC, Han S, Kang H, Kang SJ, Kim YH, Lee CW, Park SW, Hur SH, Rha SW, Her SH, Choi SW, Lee BK, Lee NH, Lee JY, Cheong SS, Kim MH, Ahn YK, Lim SW, Lee SG, Hiremath S, Santoso T, Udayachalerm W, Cheng JJ, Cohen DJ, Muramatsu T, Tsuchikane E, Asakura Y, Park SJ. Randomized trial evaluating percutaneous coronary intervention for the treatment of chronic total occlusion. Circulation 2019; 139: 1674–1683.
17. Buiten RA, Ploumen EH, Zocca P, Doggen CJM, van der Heijden LC, Kok MM, Danse PW, Schotborgh CE, Scholte M, de Man F, Linssen GCM, von Birgelen C. Outcomes in patients treated with thin-strut, very thin-strut, or ultrathin-strut drug-eluting stents in small coronary vessels: a prespecified analysis of the randomized BIO-RESORT trial. JAMA Cardiol 2019; 4: 659–669.
18. Kastrati A, Mehilli J, Dirschinger J, Dotzer F, SchüHlen H, Neumann F-J, Fleckenstein M, Pfafferott C, Seyfarth M, SchöMig A, Intracoronary stenting and angiographic results: strut thickness effect on restenosis outcome (ISAR-STEREO) trial. Circulation 2001; 103: 2816–2821.
19. Cassese S, Byrne RA, Schulz S, Hoppman P, Kreutzer J, Feuchtenberger A, Ibrahim T, Ott I, Fusaro M, Schunkert H, Laugwitz KL, Kastrati A. Prognostic role of restenosis in 10 004 patients undergoing routine control angiography after coronary stenting. Eur Heart J 2015; 36: 94–99.
20. Giacoppo D, Alfonso F, Xu B, Claessen B, Adriaenssens T, Jensen C, Perez-Vizcayno MJ, Kang DY, Degenhardt R, Pleva L, Baan J, Cuesta J, Park DW, Schunkert H, Colleran R, Kukla P, Jimenez-Quevedo P, Unverdorben M, Gao R, Naber CK, Park SJ, Henriques JPS, Kastrati A, Byrne RA. Paclitaxel-coated balloon angioplasty vs. drug-eluting stenting for the treatment of coronary in-stent restenosis: a comprehensive, collaborative, individual patient data meta-analysis of 10 randomized clinical trials (DAEDALUS study). Eur Heart J 2019;
doi: https://doi.org/10.1093/eurheartj/ehz594
21. Raber L, Yamaji K, Kelbaek H, Engstrom T, Baumbach A, Roffi M, von Birgelen C, Taniwaki M, Moschovitis A, Zaugg S, Ostojic M, Pedrazzini G, Karagiannis-Voules DA, Luscher TF, Kornowski R, Tuller D, Vukcevic V, Heg D, Windecker S. Five-year clinical outcomes and intracoronary imaging findings of the COMFORTABLE AMI trial: randomized comparison of biodegradable polymer-based biolimus-eluting stents with bare-metal stents in patients with acute ST-segment elevation myocardial infarction. Eur Heart J 2019; 40: 1909–1919.
22. Iglesias JF, Muller O, Heg D, Roffi M, Kurz DJ, Moarof I, Weilenmann D, Kaiser C, Tapponnier M, Stortecky S, Losdat S, Eeckhout E, Valgimigli M, Odutayo A, Zwahlen M, Juni P, Windecker S, Pilgrim T. Biodegradable polymer sirolimus-eluting stents versus durable polymer everolimus-eluting stents in patients with ST-segment elevation myocardial infarction (BIOSTEMI): a single-blind, prospective, randomised superiority trial. Lancet 2019; 394: 1243–1253.
23. Zaman A, de Winter RJ, Kogame N, Chang CC, Modolo R, Spitzer E, Tonino P, Hofma S, Zurakowski A, Smits PC, Prokopczuk J, Moreno R, Choudhury A, Petrov I, Cequier A, Kukreja N, Hoye A, Iniguez A, Ungi I, Serra A, Gil RJ, Walsh S, Tonev G, Mathur A, Merkely B, Colombo A, Ijsselmuiden S, Soliman O, Kaul U, Onuma Y, Serruys PW; TALENT trial investigators. Safety and efficacy of a sirolimus-eluting coronary stent with ultra-thin strut for treatment of atherosclerotic lesions (TALENT): a prospective multicentre randomised controlled trial. Lancet 2019; 393: 987–997.
24. Stone GW, Kimura T, Gao R, Kereiakes DJ, Ellis SG, Onuma Y, Chevalier B, Simonton C, Dressler O, Crowley A, Ali ZA, Serruys PW. Time-varying outcomes with the absorb bioresorbable vascular scaffold during 5-year follow-up: a systematic meta-analysis and individual patient data pooled study. JAMA Cardiol 2019;
doi: https://doi.org/10.1001/jamacardio.2019.4101
25. Bourantas CV, Zhang YJ, Garg S, Iqbal J, Valgimigli M, Windecker S, Mohr FW, Silber S, Vries T, Onuma Y, Garcia-Garcia HM, Morel MA, Serruys PW. Prognostic implications of coronary calcification in patients with obstructive coronary artery disease treated by percutaneous coronary intervention: a patient-level pooled analysis of 7 contemporary stent trials. Heart 2014; 100: 1158–1164.
26. Dini CS, Tomberli B, Mattesini A, Ristalli F, Valente S, Stolcova M, Meucci F, Baldereschi G, Fanelli F, Shlofmitz RA, Ali ZA, Di Mario C. Intravascular lithotripsy for calcific coronary and peripheral artery stenoses. EuroIntervention 2019; 15: 714–721.
27. Brinton TJ, Ali ZA, Hill JM, Meredith IT, Maehara A, Illindala U, Lansky A, Gotberg M, Van Mieghem NM, Whitbourn R, Fajadet J, Di Mario C. Feasibility of shockwave coronary intravascular lithotripsy for the treatment of calcified coronary stenoses. Circulation 2019; 139: 834–836.
27. Ali ZA, Nef H, Escaned J, Werner N, Banning AP, Hill JM, De Bruyne B, Montorfano M, Lefevre T, Stone GW, Crowley A, Matsumura M, Maehara A, Lansky AJ, Fajadet J, Di Mario C. Safety and effectiveness of coronary intravascular lithotripsy for treatment of severely calcified coronary stenoses: the disrupt CAD II study. Circ Cardiovasc Interv 2019; 12: e008434.
28. Wilson SJ, Spratt JC, Hill J, Spence MS, Cosgrove C, Jones J, Strange JW, Halperin H, Walsh SJ, Hanratty CG. Coronary intravascular lithotripsy is associated with a high incidence of “shocktopics” and asynchronous cardiac pacing. EuroIntervention 2019;
doi: https://doi.org/10.4244/EIJ-D-19-00484
29. Mehran R, Baber U, Sharma SK, Cohen DJ, Angiolillo DJ, Briguori C, Cha JY, Collier T, Dangas G, Dudek D, Dzavik V, Escaned J, Gil R, Gurbel P, Hamm CW, Henry T, Huber K, Kastrati A, Kaul U, Kornowski R, Krucoff M, Kunadian V, Marx SO, Mehta SR, Moliterno D, Ohman EM, Oldroyd K, Sardella G, Sartori S, Shlofmitz R, Steg PG, Weisz G, Witzenbichler B, Han YL, Pocock S, Gibson CM. Ticagrelor with or without aspirin in high-risk patients after PCI. N Engl J Med 2019; 381: 2032–2042.
30. Costa F, Van Klaveren D, Feres F, James S, Raber L, Pilgrim T, Hong MK, Kim HS, Colombo A, Steg PG, Bhatt DL, Stone GW, Windecker S, Steyerberg EW, Valgimigli M; PRECISE-DAPT Study Investigators. Dual antiplatelet therapy duration based on ischemic and bleeding risks after coronary stenting. J Am Coll Cardiol 2019; 73: 741–754.
31. Lopes et al. Antithrombotic Therapy after Acute Coronary Syndrome or PCI in Atrial Fibrillation. N Engl J Med 2019; 380: 1509–1524.
32. Vranckx P, Valgimigli M, Eckardt L, Tijssen J, Lewalter T, Gargiulo G, Batushkin V, Campo G, Lysak Z, Vakaliuk I, Milewski K, Laeis P, Reimitz PE, Smolnik R, Zierhut W, Goette A. Edoxaban-based versus vitamin K antagonist-based antithrombotic regimen after successful coronary stenting in patients with atrial fibrillation (ENTRUST-AF PCI): a randomised, open-label, phase 3b trial. Lancet 2019; 394: 1335–1343.
33. Gargiulo G, Goette A, Tijssen J, Eckardt L, Lewalter T, Vranckx P, Valgimigli M. Safety and efficacy outcomes of double vs. triple antithrombotic therapy in patients with atrial fibrillation following percutaneous coronary intervention: a systematic review and meta-analysis of non-vitamin K antagonist oral anticoagulant-based randomized clinical trials. Eur Heart J 2019; 40: 3757–3767.
34. Hakeem A, Ghosh B, Shah K, Agarwal S, Kasula S, Hacioglu Y, Bhatti S, Ahmed Z, Uretsky B. Incremental prognostic value of post-intervention Pd/Pa in patients undergoing ischemia-driven percutaneous coronary intervention. JACC Cardiovasc Interv 2019; 12: 2002–2014.
35. Jeremias A, Davies JE, Maehara A, Matsumura M, Schneider J, Tang K, Talwar S, Marques K, Shammas NW, Gruberg L, Seto A, Samady H, Sharp A, Ali ZA, Mintz G, Patel M, Stone GW. Blinded physiological assessment of residual ischemia after successful angiographic percutaneous coronary intervention: the DEFINE PCI study. JACC Cardiovasc Interv 2019; 12: 1991–2001.
36. Warisawa T, Cook CM, Howard JP, Ahmad Y, Doi S, Nakayama M, Goto S, Yakuta Y, Karube K, Shun-Shin MJ, Petraco R, Sen S, Nijjer S, Al Lamee R, Ishibashi Y, Matsuda H, Escaned J, di Mario C, Francis DP, Akashi YJ, Davies JE. Physiological pattern of disease assessed by pressure-wire pullback has an influence on fractional flow reserve/instantaneous wave-free ratio discordance. Circ Cardiovasc Interv 2019; 12: e007494.
37. Lee SH, Choi KH, Lee JM, Hwang D, Rhee TM, Park J, Kim HK, Cho YK, Yoon HJ, Park J, Song YB, Hahn JY, Doh JH, Nam CW, Shin ES, Hur SH, Koo BK. Physiologic characteristics and clinical outcomes of patients with discordance between FFR and iFR. JACC Cardiovasc Interv 2019; 12: 2018–2031.
38. Sen S, Ahmad Y, Dehbi HM, Howard JP, Iglesias JF, Al-Lamee R, Petraco R, Nijjer S, Bhindi R, Lehman S, Walters D, Sapontis J, Janssens L, Vrints CJ, Khashaba A, Laine M, Van Belle E, Krackhardt F, Bojara W, Going O, Harle T, Indolfi C, Niccoli G, Ribichini F, Tanaka N, Yokoi H, Takashima H, Kikuta Y, Erglis A, Vinhas H, Silva PC, Baptista SB, Alghamdi A, Hellig F, Koo BK, Nam CW, Shin ES, Doh JH, Brugaletta S, Alegria-Barrero E, Meuwissen M, Piek JJ, van Royen N, Sezer M, Di Mario C, Gerber RT, Malik IS, Sharp ASP, Talwar S, Tang K, Samady H, Altman J, Seto AH, Singh J, Jeremias A, Matsuo H, Kharbanda RK, Patel MR, Serruys P, Escaned J, Davies JE. Clinical events after deferral of LAD revascularization following physiological coronary assessment. J Am Coll Cardiol 2019; 73: 444–453.
39. DEFINE-FLAIR Trial Investigators, Lee JM, Choi KH, Koo BK, Dehbi HM, Doh JH, Nam CW, Shin ES, Cook CM, Al-Lamee R, Petraco R, Sen S, Malik IS, Nijjer SS, Mejia-Renteria H, Alegria-Barrero E, Alghamdi A, Altman J, Baptista SB, Bhindi R, Bojara W, Brugaletta S, Silva PC, Di Mario C, Erglis A, Gerber RT, Going O, Harle T, Hellig F, Indolfi C, Janssens L, Jeremias A, Kharbanda RK, Khashaba A, Kikuta Y, Krackhardt F, Laine M, Lehman SJ, Matsuo H, Meuwissen M, Niccoli G, Piek JJ, Ribichini F, Samady H, Sapontis J, Seto AH, Sezer M, Sharp ASP, Singh J, Takashima H, Talwar S, Tanaka N, Tang K, Van Belle E, van Royen N, Vinhas H, Vrints CJ, Walters D, Yokoi H, Samuels B, Buller C, Patel MR, Serruys P, Escaned J, Davies JE. Comparison of major adverse cardiac events between instantaneous wave-free ratio and fractional flow reserve-guided strategy in patients with or without type 2 diabetes: a secondary analysis of a randomized clinical trial. JAMA Cardiol 2019;
doi: https://doi.org/10.1001/jamacardio.2019.2298
40. Masdjedi K, van Zandvoort LJC, Balbi MM, Gijsen FJH, Ligthart JMR, Rutten MCM, Lemmert ME, Wilschut J, Diletti R, De Jaegere P, Zijlstra F, Van Mieghem NM, Daemen J. Validation of 3-dimensional quantitative coronary angiography based software to calculate fractional flow reserve: Fast Assessment of STenosis severity (FAST)-study. EuroIntervention 2019;
doi: https://doi.org/10.4244/EIJ-D-19-00466
41. Yu W, Huang J, Jia D, Chen S, Raffel OC, Ding D, Tian F, Kan J, Zhang S, Yan F, Chen Y, Bezerra HG, Wijns W, Tu S. Diagnostic accuracy of intracoronary optical coherence tomography-derived fractional flow reserve for assessment of coronary stenosis severity. EuroIntervention 2019; 15: 189–197.
42. Gao XF, Wang ZM, Wang F, Gu Y, Ge Z, Kong XQ, Zuo GF, Zhang JJ, Chen SL. Intravascular ultrasound guidance reduces cardiac death and coronary revascularization in patients undergoing drug-eluting stent implantation: results from a meta-analysis of 9 randomized trials and 4724 patients. Int J Cardiovasc Imaging 2019; 35: 239–247.
43. Hong MK. IVUS-XPL: 5-year outcomes from a randomized trial of intravascular ultrasound-guided vs. angiography-guided PCI of long coronary lesions. FORZA: A Randomized Trial of Fractional Flow Reserve vs Optical Coherence Tomography to Guide Revascularization of Intermediate Coronary Stenoses. Transcatheter Cardiovascular. Therapeutics; San Francisco, CA; 25–29 Sept 2019.
44. Burzotta F. Transcatheter Cardiovascular Therapeutics; San Francisco, CA; 25–29 Sept 2019.
45. Johnson TW, Raber L, di Mario C, Bourantas C, Jia H, Mattesini A, Gonzalo N, de la Torre Hernandez JM, Prati F, Koskinas K, Joner M, Radu MD, Erlinge D, Regar E, Kunadian V, Maehara A, Byrne RA, Capodanno D, Akasaka T, Wijns W, Mintz GS, Guagliumi G. Clinical use of intracoronary imaging. Part 2: acute coronary syndromes, ambiguous coronary angiography findings, and guiding interventional decision-making: an expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. Eur Heart J 2019; 40: 2566–2584.
46. Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, Agewall S, Dickstein K, Edvardsen T, Escaned J, Gersh BJ, Svitil P, Gilard M, Hasdai D, Hatala R, Mahfoud F, Masip J, Muneretto C, Valgimigli M, Achenbach S, Bax JJ; ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 2020; 41: 407–477.
47. Nagel E, Greenwood JP, McCann GP, Bettencourt N, Shah AM, Hussain ST, Perera D, Plein S, Bucciarelli-Ducci C, Paul M, Westwood MA, Marber M, Richter WS, Puntmann VO, Schwenke C, Schulz-Menger J, Das R, Wong J, Hausenloy DJ, Steen H, Berry C; MR-INFORM Investigators. Magnetic resonance perfusion or fractional flow reserve in coronary disease. N Engl J Med 2019; 380: 2418–2428.
48. Calvino-Santos R, Estevez-Loureiro R, Peteiro-Vazquez J, Salgado-Fernandez J, Rodriguez-Vilela A, Franco-Gutierrez R, Bouzas-Mosquera A, Rodriguez-Fernandez JA, Mesias-Prego A, Gonzalez-Juanatey C, Aldama-Lopez G, Pinon-Esteban P, Flores-Rios X, Soler-Martin R, Seoane-Pillado T, Vazquez-Gonzalez N, Muniz J, Vazquez-Rodriguez JM. Angiographically guided complete revascularization versus selective stress echocardiography-guided revascularization in patients with ST-segment-elevation myocardial infarction and multivessel disease: the CROSS-AMI randomized clinical trial. Circ Cardiovasc Interv 2019; 12: e007924.
49. Stone GW, Maehara A, Lansky AJ, de Bruyne B, Cristea E, Mintz GS, Mehran R, McPherson J, Farhat N, Marso SP, Parise H, Templin B, White R, Zhang Z, Serruys PW, Investigators P. A prospective natural-history study of coronary atherosclerosis. N Engl J Med 2011; 364: 226–235.
50. Bourantas CV, Garcia-Garcia HM, Torii R, Zhang YJ, Westwood M, Crake T, Serruys PW. Vulnerable plaque detection: an unrealistic quest or a feasible objective with a clinical value? Heart 2016; 102: 581–589.
51. Waksman R, Di Mario C, Torguson R, Ali ZA, Singh V, Skinner WH, Artis AK, Cate TT, Powers E, Kim C, Regar E, Wong SC, Lewis S, Wykrzykowska J, Dube S, Kazziha S, van der Ent M, Shah P, Craig PE, Zou Q, Kolm P, Brewer HB, Garcia-Garcia HM, Samady H, Tobis J, Zainea M, Leimbach W, Lee D, Lalonde T, Skinner W, Villa A, Liberman H, Younis G, de Silva R, Diaz M, Tami L, Hodgson J, Raveendran G, Goswami N, Arias J, Lovitz L, Carida Ii R, Potluri S, Prati F, Erglis A, Pop A, McEntegart M, Hudec M, Rangasetty U, Newby D. Identification of patients and plaques vulnerable to future coronary events with near-infrared spectroscopy intravascular ultrasound imaging: a prospective, cohort study. Lancet 2019; 394: 1629–1637.
52. Prati F, Romagnoli E, Gatto L, La Manna A, Burzotta F, Ozaki Y, Marco V, Boi A, Fineschi M, Fabbiocchi F, Taglieri N, Niccoli G, Trani C, Versaci F, Calligaris G, Ruscica G, Di Giorgio A, Vergallo R, Albertucci M, Biondi-Zoccai G, Tamburino C, Crea F, Alfonso F, Arbustini Eon behalf of CLIMA Investigators. Relationship between coronary plaque morphology of the left anterior descending artery and 12 months clinical outcome: the CLIMA study. Eur Heart J 2020; 41: 383–391.
53. Sheth TN, Pinilla-Echeverri N, Mehta SR, Courtney BK. First-in-human images of coronary atherosclerosis and coronary stents using a novel hybrid intravascular ultrasound and optical coherence tomographic catheter. JACC Cardiovasc Interv 2018; 11: 2427–2430.
54. Otsuka K, Villiger M, Karanasos A, van Zandvoort LJC, Doradla P, Ren J, Lippok N, Daemen J, Diletti R, van Geuns RJ, Zijlstra F, van Soest G, Dijkstra J, Nadkarni SK, Regar E, Bouma BE. Intravascular polarimetry in patients with coronary artery disease. JACC Cardiovasc Imaging 2019;
doi: https://doi.org/10.1016/j.jcmg.2019.06.015
55. Gerbaud E, Weisz G, Tanaka A, Luu R, Osman H, Baldwin G, Coste P, Cognet L, Waxman S, Zheng H, Moses JW, Mintz GS, Akasaka T, Maehara A, Tearney GJ. Plaque burden can be assessed using intravascular optical coherence tomography and a dedicated automated processing algorithm: a comparison study with intravascular ultrasound. Eur Heart J Cardiovasc Imaging 2019;
doi: https://doi.org/10.1093/ehjci/jez185
56. Ramasamy A, Ng J, White S, Johnson TW, Foin N, Girard MJA, Dijkstra J, Amersey R, Scoltock S, Koganti S, Jones D, Jin C, Raber L, Serruys PW, Torii R, Crake T, Rakhit R, Baumbach A, Mathur A, Bourantas CV. Efficacy and reproducibility of attenuation-compensated optical coherence tomography for assessing external elastic membrane border and plaque composition in native and stented segments—an in vivo and histology-based study. Circ J 2019;
doi: https://doi.org/10.1253/circj.CJ-19-0630
57. Bourantas CV, Raber L, Sakellarios A, Ueki Y, Zanchin T, Koskinas KC, Yamaji K, Taniwaki M, Heg D, Radu MD, Papafaklis MI, Kalatzis F, Naka KK, Fotiadis DI, Mathur A, Serruys PW, Michalis LK, Garcia-Garcia HM, Karagiannis A, Windecker S. Utility of multimodality intravascular imaging and the local hemodynamic forces to predict atherosclerotic disease progression. JACC Cardiovasc Imaging 2019;
doi: https://doi.org/10.1016/j.jcmg.2019.02.026
58. Costopoulos C, Maehara A, Huang Y, Brown AJ, Gillard JH, Teng Z, Stone GW, Bennett MR. Heterogeneity of plaque structural stress is increased in plaques leading to MACE: insights from the PROSPECT study. JACC Cardiovasc Imaging 2019;
doi: https://doi.org/10.1016/j.jcmg.2019.05.024
59. Gijsen F, Katagiri Y, Barlis P, Bourantas C, Collet C, Coskun U, Daemen J, Dijkstra J, Edelman E, Evans P, van der Heiden K, Hose R, Koo BK, Krams R, Marsden A, Migliavacca F, Onuma Y, Ooi A, Poon E, Samady H, Stone P, Takahashi K, Tang D, Thondapu V, Tenekecioglu E, Timmins L, Torii R, Wentzel J, Serruys P. Expert recommendations on the assessment of wall shear stress in human coronary arteries: existing methodologies, technical considerations, and clinical applications. Eur Heart J 2019; 40: 3421–3433.