The year in cardiovascular medicine 2020: acute coronary syndromes and intensive cardiac care
█ Current opinion
Authors:
Borja Ibanez1,2,3*, David Roque4, and Susanna Price5
1Centro Nacional de Investigaciones Cardiovasculares Carlos III (CNIC), Madrid, Spain; 2Cardiology Department, IIS-Fundación Jiménez Díaz University Hospital, Madrid, Spain; 3CIBERCV, Madrid, Spain; 4Cardiology Department, Prof. Dr. Fernando Fonseca Hospital, Amadora, Portugal; and 5Department of Cardiology and Department of Adult Critical Care, Royal Brompton Hospital, London, UK
Introduction
Advancements in acute cardiac care have significantly contributed to prolonging life expectancy and improving quality of care. Acute cardiac care is an area of intense basic, translational, and clinical research. In particular, acute coronary syndrome (ACS) is one of the most frequent clinical presentations requiring acute cardiac care. Despite improvements in primary prevention, the incidence of ACS and its associated mortality and morbidity remains high, with an immense impact on patients and healthcare systems. This review presents the most relevant publications in 2020 that are likely to impact on the clinical management of patients presenting with ACS requiring intensive cardiac care.
ISSUE: CARDIOLOGIA HUNGARICA | 2021 | VOLUME 51, ISSUE 4
Epidemiology of acute coronary syndromes
Identification of the association between risk factors and coronary heart disease allowed the implementation of preventive strategies. Poor control of modifiable risk factors is responsible for a large proportion of mortality and morbidity worldwide. The impact of risk factor modification was highlighted in a population analysis of 6518 men from the Seven Countries Study, in which participants were assessed over a 50-year follow-up (1). Country cohorts showing long-term decreases in risk factors had a consistent decrease of coronary heart disease mortality during follow-up. In contrast, among participants whose risk factors increased, hazard rates also increased (1). In a study of the MONICA population-based registries, all incidences of ACS in men and women aged 35–74 were recorded between 2006 and 2014 (2). Although event rates, incidence, and mortality all showed significant reductions, these were seen primarily in the 65–74 year age group, and there were no substantial declines in younger people except for mortality in young women, possibly brought about by reductions in smoking.

Racial disparities were explored in an observational cohort analysis of data from the multicentre National Cardiovascular Data Registry chest pain-MI Registry, which included 753 hospitals and 155 397 patients with acute myocardial infarction (MI) (3). Risk-adjusted 30-day readmission rates were higher in African-American patients, who had a higher prevalence of diabetes, hypertension, heart failure, bleeding risk, stroke, and peripheral arterial disease. These findings speak to the need for a more personalized consideration of genotypic and phenotypic differences in ACS.
Substantial progress has been made towards improving sex-specific ACS management (4). The incidence of acute MI has declined in the last 20 years; however, declines in MI admission have slowed in women compared with men (5). When hospitalized, women tend to be older and more deprived, and have a greater co-morbidity burden. Although more frequently managed with guideline-recommended therapy pre-admission, women less frequently receive coronary angiography and/or percutaneous coronary intervention (PCI) and are less comprehensively treated with evidence-based therapies post-MI (6, 7). An apparent paradox was revealed in the Prospective Urban Rural Epidemiological (PURE) study, which recruited 202 072 individuals aged 35–70 from 27 countries: although women less frequently received secondary prevention treatment, cardiac investigations, and coronary revascularization, they had lower 30-day mortality than men after a new cardiovascular event (8). Sex differences in ACS pathophysiology, presentation, and outcomes are presented in Figure 1.
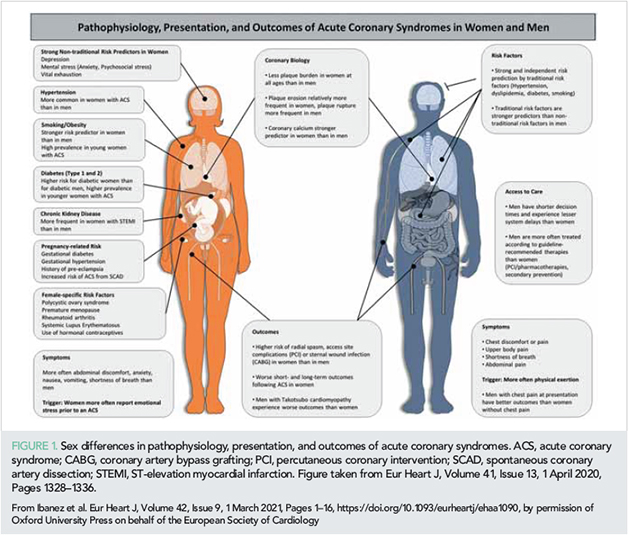
Overall, data published in 2020 illustrate that more refined strategies are needed to further reduce the burden of modifiable cardiac risk factors, with special attention to addressing sex and racial differences in the management and outcomes in ACS.
Management of non-ST segment elevation acute coronary syndrome
Diagnosis
Acute chest pain is one of the frequent reasons for attending the emergency department, and rapid diagnosis is vital (9, 10). The update of the Universal definition of MI (UDMI) has been shown to have prognostic value. Application of the fourth UDMI led to reclassification of 30% of 2302 patients presenting to the emergency department, mostly from type II MI to acute myocardial injury, and from type I MI to chronic myocardial injury (11). Importantly, reclassified patients had significantly higher rates of subsequent cardiovascular events. In a stepped-wedge cluster trial in 48 282 consecutive patients, high-sensitivity cardiac troponin (hs-cTn) and the fourth UDMI identified patients at risk of cardiovascular or non-cardiovascular events but was not associated with improved outcomes (12). Optimal management strategies and how to improve outcomes remain unknown for patients with type II MI (13, 14).
Special populations
Management of special subpopulations, such as the elderly or those with cancer, is challenging. It is increasingly recognized that invasive intervention also benefits the elderly population. In a study of 1976 NSTE-ACS patients >80 years, the adjusted cumulative 5-year mortality was 35% for those managed with invasive intervention vs. 55% for those managed with non-invasive intervention (15). A database analysis of 6 563 255 acute MI patients examined the effects of cancer on intervention and outcomes (16). Marked differences were noted, with 43.9% of cancer-free patients undergoing PCI, compared with 21% with patients with lung cancer, which had the highest in-hospital mortality. Irrespective of cancer type, metastatic disease was associated with worse outcomes, whereas historical cancer had no impact on survival. Diagnosis of active cancer is associated with conservative management and worse outcomes; however, as these parameters vary significantly according to the type and extent of disease, an individualized approach is recommended (Figure 2).
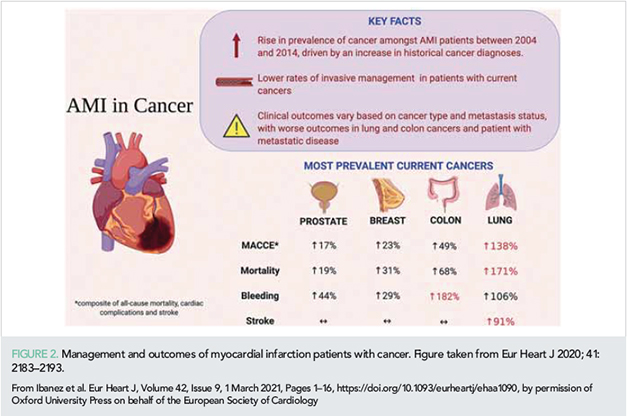
Impact of bleeding
Historical data from the SWEDEHEART study demonstrated that although the introduction of antithrombotic therapies increased bleeding events during the first year following MI, this was accompanied by a substantially greater reduction in ischaemic events and an increased survival (17). In contrast, analysis of a harmonized dataset from four multicentre randomized controlled trials (RCTs) comprising 45 011 participants found that post-discharge bleeding after an ACS was associated with a similar increase in subsequent all-cause mortality and had a similar prognostic impact to post-discharge MI (18). These apparently conflicting data suggest that antithrombotic therapy overall has a clear benefit but bleeding identifies a population at higher risk of mortality.
Management of ST-segment elevation myocardial infarction
One of the most rapidly advancing areas of cardiology is STEMI. Since the 2017 ESC STEMI guidelines, important data with implications for patient management have continued to appear, and 2020 is a particularly prolific year in this regard.
Reperfusion strategies
The landmark DANAMI-2 and PRAGUE-2 trials demonstrated that transfer to the catheterization lab was superior to immediate fibrinolysis (19). 2020 saw the publication of the very long-term follow-up of the DANAMI-2 RCT (20). After 16 years of follow-up, the composite of death or MI remained significantly lower in patients transferred to PCI than in those undergoing on-site fibrinolysis. This is the first time that primary PCI has been shown to be associated with lower cardiac mortality than stand-alone fibrinolysis in a trial. The routine performance of angiography within 24 h after fibrinolysis has significantly reduced the rates of re-MI and future coronary revascularizations. Indeed, in the STREAM trial, which compared transfer to PCI vs. onsite fibrinolysis followed by routine angiography, cardiac mortality at 1 year was similar for both treatment strategies (19). A new analysis of 2942 patients from the French FAST-MI registry found that the 5-year survival was lower in patients undergoing late PCI (>120 min) than in those undergoing timely PCI (within 120 min of diagnosis) or immediate fibrinolysis (21).
Triage of patients to the appropriate reperfusion strategy requires the presence of well-trained healthcare providers on the scene and the integration of emergency medical services within an organized network. The creation of pan-European registries is critical to the acquisition of continuous information in this regard (22). Clinical guidelines recommend regular monitoring and feedback in order to maintain a high quality of care, but there are few quantifiable data supporting this strategy. In a recent paper, the prospective, multicentre FITT-STEMI study assessed the long-term impact of formalized data assessment and systematic feedback on performance and mortality (23). Over its 10-year evaluation, FITT-STEMI recorded significant improvement in all performance quality indicators used for feedback, and this feedback-informed continuous improvement in key quality indicators was linked to a significant reduction in mortality (23).
Vascular access during primary percutaneous coronary intervention
The superiority of radial over femoral access seemed to be set in stone, and yet a recent RCT has shown intriguing results. The SAFARI-STEMI was a multicentre, open-label, RCT with blinded endpoint adjudication undertaken over 7 years (2011–2018) at five high-volume PCI centres in Canada (24). STEMI patients were randomized 1:1 to radial vs. femoral access. The trial was stopped after enrolment of 2292 patients (47% of the original sample size) on the grounds of futility. In the trial, 30-day all-cause mortality was 1.5% vs. 1.3% in the radial and femoral access groups, respectively (P = 0.69). Intriguingly, bleeding outcomes (which were very few) did not differ between groups. It should be noted that a vascular closure device was used in 68% of patients assigned to femoral access. Whereas the SAFARI-STEMI trial assessed highly selected centres and operators, the pivotal MATRIX trial (25) was closer to the real-world clinical care, with 78 centres of different volumes in four countries. Therefore, while the SAFARI-STEMI trial shows that femoral access performed by operators experienced in the use of closure devices is a good alternative to radial access, these data should not modify the recommendation for radial access as the default vascular access route, as recommended in ESC guidelines (10, 26).
Management of non-culprit lesions
Clinical benefits of complete revascularization
Multivessel disease (MVD) is present in >50% of STEMI patients. Five major trials (Figure 3) published in recent years changed the therapeutic approach to severe stenosis in the non-infarct-related artery (IRA). The 2017 ESC STEMI guidelines introduced a major change, recommending that non-IRA preventive PCI should be considered before hospital discharge. Since then, this topic has been the subject of the large COMPLETE trial (27) and several meta-analyses. Two meta-analyses from 2020 (28, 29) clearly demonstrate that non-IRA preventive PCI, performed within weeks of the index STEMI, is associated with lower cardiovascular mortality. A pre-specified subanalysis of the COMPLETE trial concluded that complete revascularization reduced major cardiovascular outcomes to a greater extent in patients with more severe stenosis [≥60% on quantitative coronary angiography (QCA)] (30). A similar finding was recently reported after analysis of data from the Compare-acute trial (31). The authors related events in patients allocated to medical treatment (IRA-only PCI) to the fractional flow reserve (FFR). Non-IRAs that required subsequent revascularization had a lower FFR than those without events. Increased risk of major adverse cardiovascular events (MACE) was significantly higher for lesions with FFR below 0.80 (31).
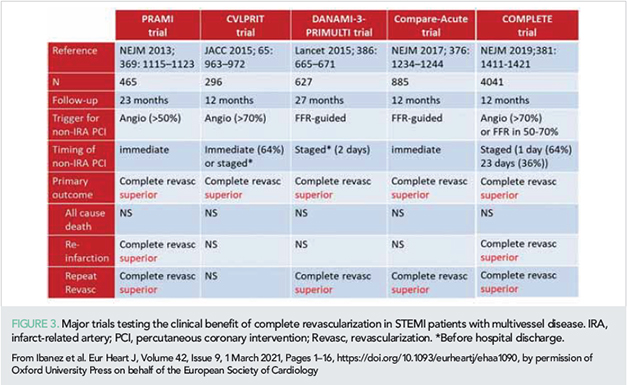
How to identify non-IRA severe lesions benefiting from PCI
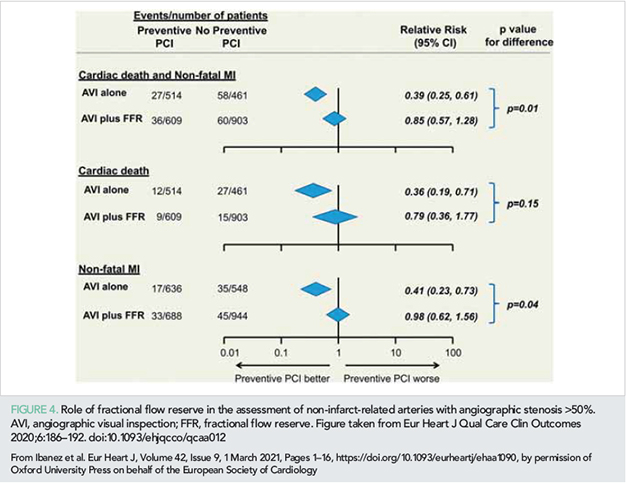
There is no consensus about which method is more suitable for cataloguing a non-IRA as a candidate for preventive PCI in STEMI patients [angiography (visual inspection), FFR, or FFR after intermediate lesions on angiography]. Two recent studies intriguingly suggested that angiography-guided but not FFR-guided non-IRA PCI is associated with reduced major adverse events in STEMI patients with MVD (32, 33). Wald et al. performed a meta-analysis of 10 RCTs (3031 patients in total) and assessed outcomes in patients with complete revascularization vs. IRA-only PCI according to whether the decision to carry out non-IRA preventive PCI was based on angiography alone or on angiography plus FFR (32). The authors concluded that preventive PCI of the non-IRA was associated with a significant reduction in cardiac death and non-fatal MI only when the decision to proceed with non-IRA PCI was based solely on angiography (Figure 4) (32). Similar findings were reported in an independent study by Gallone et al (33). Here, the authors conducted an independent meta-analysis of seven RCTs, including a total of 6597 patients. The patients were stratified according to the strategy used to guide PCI of non-IRA lesions in the complete revascularization arm: angiography-guided (≥70% diameter stenosis) vs. FFR-guided (≤0.80 for lesions with ≤90% diameter stenosis). The authors found that angiography-guided but not FFR-guided complete revascularization was associated with less recurrent MI (33). Conversely, both strategies were associated with fewer repeat revascularizations (33). None of these studies evaluated the specific question on an ad hoc basis (34), and these data should therefore be interpreted with caution; nevertheless, these two independent meta-analyses suggest that in STEMI patients with angiography-confirmed severe stenosis in a non-IRA, PCI should be performed regardless of the FFR result.
Ischaemia vs. vulnerable characteristics of non-IRA lesions
The accuracy of FFR to defer preventive PCI in arteries with severe angiography-detected stenosis has been questioned for patients with ACS. On one hand, coronary physiology in ACS might vary from that in stable patients, while, on the other hand, intermediate lesions with negative FFR in ACS patients might have vulnerable features that make them more prone to future rupture. Indeed, in a recently published study including data from 12 844 ACS patients from the TRITON-TIMI 38 study, spontaneous events in non-culprit lesions predominated 30 days after the index event (35). Enlightening results from the Optical Coherence Tomography substudy of the COMPLETE trial show that 50% of assessed patients had at least one lesion in a non-IRA with features of a complex vulnerable plaque (36).
In summary, a significant amount of data published in 2020 has increased our understanding of the implications of severe non-IRA lesions in STEMI patients and the best way to deal with them. Severe lesions on angiography seem to benefit from PCI without further FFR inspection. A more comprehensive description of the topic can be found in a major review recently published in the journal (37).
Cardioprotection during STEMI
During ischaemia, necrosis progresses from the endocardium to the epicardium. A new cardiac magnetic resonance (CMR) clinical study this year demonstrated that the wave front of necrosis progression moves in both transmural and lateral directions (Figure 5) (38). These data have important implications because cardioprotective strategies can salvage myocardium transmurally and laterally, potentially having a strong benefit in terms of global systolic function. Final infarct size is the result of several interconnected mechanisms (39). There is growing evidence that these mechanisms are modified by ageing (40), making the identification of therapeutic targets more challenging.
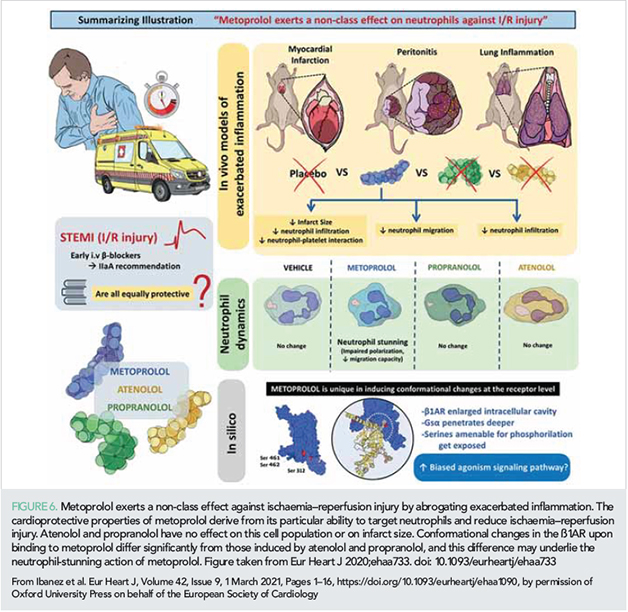
In >50% of patients, efficient myocardial perfusion is not achieved despite the unblocking of the epicardial coronary artery, and this is mostly due to severe microvascular obstruction (MVO) (41). Several interventions targeting MVO have been tested in experimental and clinical studies (39). Among them, one of the strategies with more encouraging results is the early administration of the β1-selective blocker metoprolol (42). Metoprolol injection in patients undergoing primary PCI is associated with less CMR-measured MVO (42). A very recent experimental study demonstrated that this cardioprotective ability is not shared by other beta-blockers. Metoprolol, but not the other beta-blockers tested, reduces infarct size by stunning neutrophils during reperfusion, resulting in less MVO (43). In silico modelling suggests that metoprolol induces a differential conformational change in the β1-adrenergic receptor that seems to trigger a biased agonistic effect (Figure 6) (43). In addition to reducing reperfusion injury, i.v. administration of metoprolol early in the course of ongoing MI is able to blunt the time-dependent progression of infarct size in a large animal model (44) (Figure 5). Reduced MVO was also the focus of a substudy of the small MRUSMI trial; 100 STEMI patients were randomized 1:1 to control or the novel intervention sonothrombolysis (high mechanical index impulses from a diagnostic ultrasound transducer during an i.v. microbubble infusion). The primary report had already shown an association of sonothrombolysis with a smaller infarct size (45). The new substudy shows that sonothrombolysis protected against MVO and improved global longitudinal strain in patients with an occluded artery on initial angiography (46).
Reducing time to treatment is a central tenet of acute MI management that aims to limit mortality, infarct size, and the development of heart failure (47). Following the pilot STEMI-DTU study, which suggested a role for left ventricular (LV) unloading in limiting infarct size (48), a series of mechanistic studies in a pre-clinical pig model have examined LV unloading prior to revascularization. This analysis demonstrated that transvalve unloading [not extracorporeal membrane oxygenation (ECMO)] limits myocardial injury before reperfusion, reduces infarct size, and preserves myocardial energy substrate levels and mitochondrial structure and function in the infarct zone. While these findings need confirming in patient cohorts with clinical endpoints, they provide novel insights into ischaemia-reperfusion and serve as a salutary reminder that not all mechanical circulatory support devices are the same (see section below).
The cardioprotective strategy includes measures to reduce malignant arrhythmias during the acute phase of STEMI. The incidence of severe ventricular arrhythmia during STEMI is reduced by early i.v. administration of beta-blockers (49), an effect mediated by epinephrine blockade not only in cardiomyocytes but also in cardiac-resident macrophages (50). However, in some patients, malignant arrhythmias occur despite beta-blocker administration. A recent translational study demonstrated that patients developing primary ventricular fibrillation during an ongoing MI had higher circulating levels of the co-transmitter neuropeptide Y (NPY) than matched patients without malignant arrhythmias (51). Experimental analysis in the same study demonstrated that NPY release from stimulation of stellate ganglia reduced the threshold for ventricular fibrillation despite the administration of beta-blockers. Pharmacological blockade of the NPY receptor Y1 prevented the development of malignant arrhythmias. These results identify Y1 as a novel therapeutic target for drugs acting in synergy with beta-blockers to prevent ventricular arrhythmias during ongoing STEMI (51).
Pharmacological agents for acute coronary syndromes
The ever-growing maze of antiplatelet therapy
DAPT vs. P2Y12 monotherapy after PCI
In the TICO trial, 3056 patients with ACS undergoing PCI were randomized 1:1 to ticagrelor monotherapy after 3 months of dual antiplatelet therapy (DAPT) vs. standard DAPT (aspirin + ticagrelor for 12 months). Ticagrelor monotherapy after 3 months was associated with a significant reduction in the composite primary endpoint of 1-year net adverse clinical events (2% absolute reduction) (52). In a pre-specified subanalysis of the diabetic cohort in the TWILIGHT study, ticagrelor monotherapy after 3 months was associated with a reduced risk of clinically relevant bleeding without any increase in ischaemic events, consistent with the main results of the trial (53). Another pre-specified subanalysis of the TWILIGHT study showed that the benefits of shorter DAPT were also seen in the subpopulation undergoing complex PCI (54). The benefits of ticagrelor monotherapy after 3 months are more pronounced in patients presenting with NSTEMI (55). These results, suggesting a reduced risk of bleeding events with shorter DAPT without an increased risk of ischaemic events, are in line with other recently reported studies (including SMART-CHOICE (56), STOPDAPT-2 (57), and GLOBAL-LEADERS (58) and with a meta-analysis including trials in which aspirin was dropped 1–3 months after PCI (59). Conversely, the RENAMI registry showed that prolonged DAPT (>12 months) with potent P2Y12 inhibitors had a beneficial effect on ischaemic events (offsetting the increased risk of higher bleeding) except in patients older than 75 years and in women (60). Moreover, a pre-specified subanalysis within the PEGASUS-TIMI 54 trial showed that patients with prior ACS (1–3 years before) benefitted from long-term ticagrelor on top of aspirin (fewer ischaemic events) regardless of whether they had prior coronary stenting (61).
In the recent HOST-REDUCE-POLYTECH-ACS trial, 2338 ACS patients receiving DAPT with prasugrel for 1 month were randomized to half-dose prasugrel (5 mg daily) DAPT or full dose (10 mg) DAPT for an additional 11 months. Prasugrel-based dose de-escalation was associated with a net clinical benefit driven by a reduction in bleeding without an increase in ischaemic events (62).
Prasugrel vs. ticagrelor in ACS patients
According to the new ESC NSTEMI guidelines (10), prasugrel should be considered in preference to ticagrelor for NSTE-ACS patients who proceed to PCI. This notable recommendation change is mainly based on the results of the multicentre open-label ISAR-REACT 5 trial (63). As the trial was designed to demonstrate that ticagrelor would be associated with fewer adverse events, the conclusion that prasugrel performed better generated some controversy. In a pre-specified subanalysis of the ISAR-REACT 5 trial STEMI population (41% of the sample), no significant differences in the primary endpoint (composite of 1-year death, MI, or stroke) were found between prasugrel and ticagrelor, albeit the latter was associated with a higher incidence of recurrent MI (64). Conversely, in a post-hoc analysis of the trial undertaken in the NSTEMI population (59% of the sample), prasugrel was superior to ticagrelor in reducing the primary endpoint without increasing the risk of bleeding (65).
In line with the ISAR-REACT 5 results, a small mechanistic study showed that, compared with ticagrelor and clopidogrel, prasugrel administered pre-PCI is associated with improved endothelial function, stronger platelet inhibition, and lower interleukin-6 (IL-6) levels, thus limiting stent-induced endothelial dysfunction and inflammation (66). However, a recent meta-analysis of 12 trials found that of the three P2Y12 receptor inhibitors, only ticagrelor was associated with decreased mortality (67). A more recent large study of three databases including 31 290 ACS patients undergoing PCI found no differences in net adverse clinical events between patients taking ticagrelor or clopidogrel (68).
Pre-loading strategies
Another new addition to the guidelines on NSTE-ACS is the recommendation against routine pre-treatment with a P2Y12 receptor inhibitor in patients with unknown coronary anatomy who are scheduled for early invasive management (10). In line with this recommendation, in the DUBIOUS trial, pre-loading with ticagrelor had no benefit in NSTE-ACS patients (69). After an interim analysis of 1449 patients, the trial was prematurely interrupted for futility reasons (low incidence of the primary outcome and minimal differences between groups) (69).
Systemic platelet inhibition strategies
Although glycoprotein (GP) IIb/IIIa receptor inhibitors are now only recommended for bail-out situations, the small FABOLUS-FASTER trial randomized 122 P2Y12-naive STEMI patients 1:1:1 to cangrelor infusion followed by prasugrel, tirofiban infusion followed by prasugrel, or prasugrel (chewed or integral). At 30 min, tirofiban yielded superior inhibition of platelet aggregation (primary endpoint) compared with cangrelor, and both were superior to chewed prasugrel (which did not provide superior platelet inhibition compared with the integral form) (70). The new kid on the block is selatogrel, a new highly selective, reversible P2Y12 inhibitor with a fast onset of action. In a phase II trial, a single subcutaneous administration of selatogrel to MI patients reached maximum plasma concentration at ~1 h (with profound platelet inhibition as early as 15 min), without major bleeding complications (71).
Overall, these data identify monotherapy with potent P2Y12 inhibitors as a valid alternative to classical DAPT after the early post-MI period. While prasugrel is recommended over ticagrelor as the P2Y12 inhibitor of choice after an MI, there are still contradictory data. De-escalation of prasugrel dose after 1 month appears as a valid alternative that can benefit patients at high bleeding risk. Cumulative evidence shows that pre-loading with P2Y12 inhibitors in ACS patients undergoing early invasive management does not offer benefits. When fast platelet inhibition is needed, tirofiban seems a good option, with s.c. selatogrel being a promising alternative.
Personalized treatment after acute coronary syndrome
Genotyping
The GIANT study determined the CYP2C19 genotype in saliva samples from 1445 STEMI patients within 4 days after PCI to allow appropriate treatment adjustment (72). Carriers of loss-of-function (LOF) alleles (22% of the study population) received prasugrel or a double dose of clopidogrel (potent thienopyridine strategy), while patients with wild-type or gain-of-function alleles were treated according to investigator preference. After genotyping, the potent strategy was prescribed to 99% of LOF carriers and to 55% of the other patients. Patients with LOF alleles showed no difference from the other patients in ischaemic or bleeding events at 1 year (72). The larger TAILOR PCI trial (5302 patients undergoing PCI, 50% ACS) failed to show any ability of a CYP2C19 genotype-guided strategy to reduce adverse cardiovascular events (73). Another study, in which a polygenic response score was derived from several CYP2C19 polymorphisms, showed that the number of alleles associated with increased platelet reactivity is a key determinant of clinical outcomes (74).
Age and renal function
The POPular-AGE open-label trial randomized 1002 NSTE-ACS patients older than 70 years to clopidogrel or prasugrel/ticagrelor, and found that the trade-off between ischaemic and bleeding events favoured clopidogrel (75). Similarly, in a SWEDEHEART registry report on ACS patients aged ≥80 years, ticagrelor was associated with a higher risk of bleeding and death, without providing any additional reduction in ischaemic outcomes (76). Data from the RENAMI and BLEEMACS registries showed that prasugrel and ticagrelor performed better than clopidogrel at reducing the risk of all-cause mortality and recurrent MI, without an increase in major bleeding, in ACS patients with chronic kidney disease [estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2) treated by PCI (77).
Altogether, these data show that while some patient subsets clearly benefit from potent P2Y12 inhibitors (i.e. those with renal failure), others may not (i.e. the elderly). Tailored antithrombotic therapy based on genotype does not seem to offer clinical benefit yet.
Looking at old drugs with new eyes
Systemic inflammation is increasingly recognized as a therapeutic target for atherothrombosis. In a recent experimental study, colchicine was shown to stabilize atherosclerotic plaques (78). In the landmark COLCOT trial of 4745 patients within 1 month after MI, low-dose colchicine (0.5 mg once daily) was associated with a significant reduction in the primary efficacy endpoint, mainly driven by significant reductions in stroke and urgent hospitalization for angina leading to coronary revascularization (79). The benefit seems to be stronger when colchicine is initiated within the first 3 days after MI (80). In the small COLCHICINE-PCI trial, acute oral colchicine (1.8 mg) before PCI had no effect on the risk of PCI-related myocardial injury, although it attenuated the increase in IL-6 and high-sensitivity C-reactive protein (hsCRP) (81). The benefits of colchicine have recently been shown to extend to patients with chronic coronary artery disease. In the LoDoCo2 trial enrolling 5522 patients (84% with prior ACS), 0.5 mg/day colchicine was associated with a reduced incidence in the composite primary endpoint (cardiovascular death, spontaneous MI, ischaemic stroke, or ischaemia-driven coronary revascularization) but did not significantly decrease cardiovascular deaths and was associated with a numerical increase in non-cardiovascular deaths (82). The increase in non-cardiovascular deaths was also reported in the small Australian COPS trial, which randomized 795 ACS patients to placebo or colchicine (0.5 mg twice daily for the first month, then 0.5 mg daily for 11 months) (83). Colchicine was not associated with a reduction in the primary outcome of ischaemic events, but was associated with a higher rate of all-cause mortality, mainly non-cardiovascular (83).
Altogether, these data identify colchicine as therapy that might be considered for post-MI patients with high residual ischaemic risk.
The benefits of chronic beta-blocker use in post-MI patients is well established for those with reduced LV ejection fraction (LVEF), but the evidence is less firm for other patients. A recent study of the Korean national database followed 28 970 post-MI patients who were event-free after 1 year. Continuation with beta-blockers beyond 1 year was associated with a significantly lower rate of all-cause death than when therapy was discontinued before 1 year (84). The benefits of beta-blockers were maintained beyond 2 years but not beyond 3 years (84). Although this registry study was rigorous, it has important limitations that preclude a definitive answer to the question of post-MI beta-blocker therapy for patients with preserved EF (85). In Europe, five ongoing trials are testing the role of beta-blockers in post-MI patients without reduced EF (REBOOT-CNIC, REDUCE-SWEDEHEART, BETAMI, DANBLOCK, and ABYSS). These trials will pool >20 000 properly randomized patients. The results of these trials will provide a definitive answer to this highly relevant question.
Critical care for high-risk acute coronary syndromes
The most lethal complications of MI remain cardiac arrest (CA) and cardiogenic shock (CS). CS complicates between 5% and 15% of STEMIs and is associated with in-hospital and 6-year mortality rates of 40–45% and 69%, respectively (86). In a regional STEMI programme, CS and CA affected 9% and 11% of the 4511 patients, respectively, but represented 76% of in-hospital deaths (87). The importance of CA as a disease modifier in CS is evident from comparison of in-hospital mortality data (CS+ and CA+, 44% vs. CS+ and CA–, 23%; P < 0.001). After discharge, the 5-year survival probability for CS patients was 0.69 and for CA patients was 0.89. The prognosis of CA patients was determined by the cardiac rhythm at presentation, and CS+ patients remained at high risk of lethal events (87). A recent retrospective study has shown that young women with CS complicating an MI are treated less aggressively and experience higher in-hospital mortality than men (88).
MI patients with concurrent CS are increasingly given mechanical circulatory support. This trend was explored in a controversy-provoking, registry-based retrospective cohort study of 168 propensity-matched patient pairs that compared the Impella heart pump with intra-aortic balloon pumps (IABPs) (89). The risks of in-hospital death and bleeding were significantly higher in patients supported with the Impella pump (45.0% vs. 34.1% and 31.3% vs. 16.0%, respectively). However, direct comparison of complication rates with different devices would require high-quality RCTs powered for hard clinical endpoints.
A recent observational study has shown that LV unloading with Impella is associated with lower mortality in patients with CS treated with venoarterial ECMO (90).
In the very small phase II ARREST trial, 30 patients with out-of-hospital CA and refractory ventricular fibrillation were randomized to ECMO-facilitated resuscitation or standard treatment. Six-month survival was significantly better in the early ECMO group (91).
Randomized clinical trials are needed to confirm the best strategy management for patients presenting with CS ± CA complicating an MI.
Atypical forms of myocardial infarction: from coronary dissection to spasm
The most typical form of STEMI is the formation of an occluding thrombus on a ruptured atherosclerotic plaque (type I MI). However, emergency angiography sometimes shows other findings, from MI with non-obstructive coronary arteries (MINOCA) to spontaneous coronary artery dissections (SCADs). Diagnosis, treatment, and prognosis of these patients are less well established. Of the 276 522 MI elderly patients (≥65 years old) in the US National Cardiovascular Data Registry CathPCI Registry, 16 849 (6%) fulfilled MINOCA criteria (92). Compared with MI patients with obstructive coronary artery disease, patients with MINOCA had a lower 1-year rate of all-cause death (12% vs. 17%) and lower incidence rates of re-MI (1% vs. 6%) and heart failure (6% vs. 9%) (92). While this study shows that elderly patients with MINOCA have a relatively high incidence of 1-year MACE, this rate is significantly better than that of patients with typical MI.
MINOCA can also be caused by vasomotor dysfunction including epicardial and microvascular coronary spasm. Accurate diagnosis requires the execution of a provocative test (intracoronary acetylcholine testing), but the safety of this test in the acute MI setting has been questioned. A single-centre 10-year experience in performing provocative tests (80 MINOCA and 100 stable angina patients) has been reported (93). Epicardial spasm was found more frequently in MINOCA patients than in stable angina patients (35% vs. 19%). Conversely, microvascular spasm was more frequent in stable angina patients (53% vs. 29% in MINOCA). Importantly, the rate of side effects was relatively low (15%), and that of complications (always reversible) was very low (2.2%) and did not differ between MINOCA and stable angina patients (93).
SCAD is another entity that has gained attention in recent years. In the US Nationwide Readmissions Database, which included 2.5 million patients diagnosed with MI, 1386 (0.05%) were diagnosed with SCAD (94). Compared with typical MI patients, patients with SCAD had a higher incidence of 30-day readmission (12% vs. 10%). In the SCAD population, 81% of readmissions were due to cardiac causes. The most frequent cardiac cause was reinfarction (45%), followed by chest pain (20%) and arrhythmia (13%). Half of SCAD readmissions occurred in the first week post-discharge, and more than half of reinfarctions occurred in the first 2 days post-discharge (94). A recent report investigated the long-term impact of SCAD on CMR-measured myocardial function in 158 SCAD survivors (98% female) (95). The mode of presentation was NSTEMI in 60%, STEMI in 33%, and cardiac arrest in 7%. Most SCAD patients had no or small infarctions and preserved RF on CMR performed >1 year after the index event. Larger infarctions on CMR were associated with STEMI presentation, TIMI 0/1 flow, multivessel SCAD, and the presence of connective tissue disorders (95).
In summary, recent publications add new information about the prognosis of elderly patients presenting MINOCA. Performance of provocative tests in MINOCA patients is safe and in a non-trivial proportion of them identify epicardial spasm as its causal mechanism. Compared with typical MI, SCAD is associated with a high rate of early readmissions.
Acute coronary syndromes during the coronavirus disease 2019 pandemic
The year 2020 will be remembered as the year of the COVID-19 pandemic. The COVID-19 crisis has had a major impact on the management, treatment, and prognosis of ACS patients (96). Dedicated reviews and position papers have detailed the impact of COVID-19 on cardiovascular disease in general. Here, we want to briefly highlight the most important data on the impact of COVID-19 on ACS. Most notably, the European Association of Percutaneous Cardiovascular Interventions and the Acute Cardiovascular Care Association published a dedicated joint position statement on the invasive management of ACS during the COVID-19 pandemic in May 2020 (97).
The first noticed impact of COVID-19 was the significant reduction in hospital admissions for ACS during the first wave of the COVID-19 crisis in Europe (March–April) compared with similar periods in previous years. This reduction was consistently reported in several European countries, including Spain (98), Italy (99), Austria (100), the UK (101) and others (102). A recent ESC survey covering >140 countries worldwide showed that the COVID-19 crisis has had a major effect not only on the number of STEMI presentations (significantly reduced) but also on the rate of delayed presentations (significantly higher) (103).
During the first wave of the COVID-19 crisis, the entire healthcare system (hospitals, emergency medical services, etc.) underwent a massive reorganization to deal with the overwhelming number of infection-related admissions (104). This reorganization involved rapid structural adaptations (networks, spoke, and hub centres) and therapeutic adjustments (104).
SARS-CoV-2 infection is associated with a highly thrombogenic status. Autopsy studies show that COVID-19 patients frequently have thrombo-embolic disease (105). This appears to be reflected in the apparent association of anticoagulation with better clinical outcomes in patients admitted for COVID-19 (105). Several studies have demonstrated that STEMI patients with COVID-19 have a significantly higher thrombus burden in culprit lesions (106, 107) and a higher incidence of multivessel thrombosis (106). This has resulted in higher heparin doses to achieve therapeutic activated clotting times and a higher use of GP IIb/IIIa receptor inhibitors. Importantly, STEMI patients with concurrent COVID-19 have a higher incidence of stent thrombosis (106). Mortality of patients admitted for ACS with concurrent COVID-19 seems to be significantly higher than that of contemporaneous ACS patients without infection (107).
Myocardial injury, evidenced as an elevation of troponins, is found in 10–35% of patients hospitalized with COVID-19 (108). In a study of 100 patients recovered from severe COVID-19, 60% had some evidence of myocardial inflammation on CMR (109). While lymphocytic myocarditis has been shown in 14% of cases in a systematic evaluation of autopsies of COVID-19 patients (110), current evidence suggests that SARS-CoV-2 cardiac infection is uncommon (111). In most COVID-19 patients, myocardial injury is secondary to a myocardial oxygen supply/demand disbalance in the context of critical illness (especially i+n patients with pre-existing cardiovascular disease) and to a systemic cytokine storm.
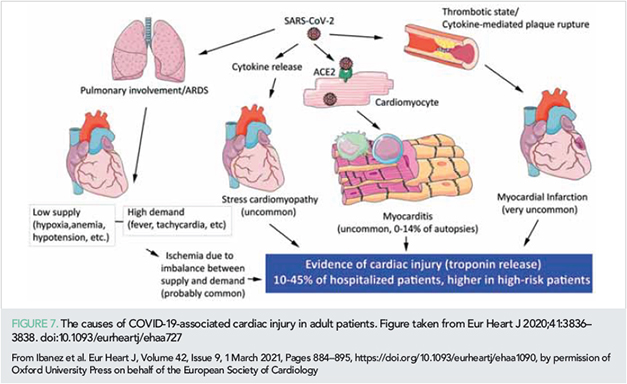
Figure 7 summarizes the mechanisms leading to myocardial injury in patients with COVID-19.
Post-acute coronary syndrome myocardial healing
As discussed above, final infarct size (the extent of irreversible myocardial loss) is the main determinant of long-term mortality and morbidity, and infarct size can be limited by acute interventions during ongoing STEMI. However, there is a lack of therapies able to restore cardiac function after the acute episode, when the infarction is complete and necrotic myocardium is replaced by fibrotic tissue. The ability of cell therapy to improve outcomes in patients with large infarctions has been a matter of intense research over the past 15 years. This year, the results of two large cell therapy trials have been published. The BAMI trial enrolled 375 STEMI patients with low LVEF who were randomized to control or intracoronary infusion of autologous bone marrow-derived mononuclear cells 2–8 days after primary PCI (112). The main outcome of this ambitious trial was all-cause death, which did not differ between groups (3.3% and 3.8%) (112). The incidence of 2-year mortality was overtly below that expected in the trial design (12%), and the results should thus be interpreted with caution. The ALLSTAR trial enrolled 142 patients 1–12 months after MI with low LVEF and a large scar. These patients were randomized 2:1 to placebo or intracoronary infusion of allogeneic cardiac progenitor cells (cardiosphere-derived cells; CDCs) (113). The primary efficacy endpoint (change in CMR-measured infarct size at 1 year) did not differ between groups. LV volume was reduced in the cell therapy group (113).
Despite the disappointing results of both studies, they confirm the safety of intracoronary administration of cell therapy at different timings after MI. A crucial obstacle to moving this field forward is the identification of the target population that would benefit from these advanced therapies.
Outlook
In summary, 2020 has witnessed important studies that should have an impact on acute cardiac care management. Despite great advance in preventive strategies, the burden of modifiable risk factors is still very high, with sex and racial differences in the management and outcomes in ACS. Management of ACS patients with concurrent cancer is associated with a more conservative management and worse outcomes. The updated (fourth) UDMI results in a reclassification of a significant proportion of patients in a different MI type, coming with prognostic implications. 2020 observed the confirmation that complete revascularization is clearly the best strategy for stable STEMI patients with multivessel disease. The search of co-adjuvant therapies that might reduce infarct size in STEMI patients is still very active. Metoprolol has been shown to exert unique non-class cardioprotective effects and thus appears as the beta-blocker of choice in STEMI patients. The best antiplatelet regimen is a field of very active research. A more personalized approach results in better outcomes. The old and inexpensive drug colchicine has been revealed as a good candidate for post-MI patients with high residual risk. Myocardial injury has been shown to be frequent in patients with severe COVID-19, but this seems more related to the general condition of the patient than to direct cardiac viral infection. In addition, SARS-CoV-2 infection is associated with a high thrombotic burden. The very active clinical and translational research in the field of acute cardiac care will result in a continuous update on this topic.
Funding
B.I. is supported by the European Commission (ERC-CoG grant no. 819775, and European Regional Development Fund no. AC16/00021), the Spanish Ministry of Science and Innovation (MICINN; ‘RETOS 2019’ grant no. PID2019-107332RB-I00), the Instituto de Salud Carlos III (ISCIII; PI16/02110, and DTS17/00136), and the Comunidad de Madrid (S2017/BMD-3867 RENIM-CM). The CNIC is supported by the ISCIII, the MICINN, and the Pro CNIC Foundation.
Conflict of interest: none declared.
References
1. Menotti A, Puddu PE, Kromhout D, et al. Coronary heart disease mortality trends during 50 years as explained by risk factor changes: the European cohorts of the Seven Countries Study. Eur J Prev Cardiol 2020;27: 988–998.
2. Meirhaeghe A, Montaye M, Biasch K, et al. Coronary heart disease incidence still decreased between 2006 and 2014 in France, except in young age groups: results from the French MONICA registries. Eur J Prev Cardiol 2020;27:1178–1186.
3. Pandey A, Keshvani N, Khera R, et al. Temporal trends in racial differences in 30-day readmission and mortality rates after acute myocardial infarction among Medicare beneficiaries. JAMA Cardiol 2020;5:136–145.
4. Haider A, Bengs S, Luu J, et al. Sex and gender in cardiovascular medicine: presentation and outcomes of acute coronary syndrome. Eur Heart J 2020;41:1328–133.
5. Mefford MT, Li BH, Qian L, et al. Sex-specific trends in acute myocardial infarction within an integrated healthcare network, 2000 through 2014. Circulation 2020;141:509–519.
6. Jackson AM, Zhang R, Findlay I, et al. Healthcare disparities for women hospitalized with myocardial infarction and angina. Eur Heart J Qual Care Clin Outcomes 2020;6:156–165.
7. DeFilippis EM, Collins BL, Singh A, et al. Women who experience a myocardial infarction at a young age have worse outcomes compared with men: the Mass General Brigham YOUNG-MI registry. Eur Heart J 2020;41:4127–4137.
8. Walli-Attaei M, Joseph P, Rosengren A, et al. Variations between women and men in risk factors, treatments, cardiovascular disease incidence, and death in 27 high-income, middle-income, and low-income countries (PURE): a prospective cohort study. Lancet 2020;396:97–109.
9. Stepinska J, Lettino M, Ahrens I, et al. Diagnosis and risk stratification of chest pain patients in the emergency department: focus on acute coronary syndromes. A position paper of the Acute Cardiovascular Care Association. Eur Heart J Acute Cardiovasc Care 2020; 9:76–89.
10. Collet JP, Thiele H, Barbato E, et al. ESC Scientific Document Group. 2020 ESC Guidelines for the management of acute coronary syndromes in patients pre-senting without persistent ST-segment elevation. Eur Heart J 2020; doi: https://doi.org/10.1093/eurheartj/ehaa575
11. Hartikainen TS, Sorensen NA, Haller PM, et al. Clinical application of the 4th Universal Definition of Myocardial Infarction. Eur Heart J 2020;41:2209–2216.
12. Chapman AR, Adamson PD, Shah ASV, et al. High-sensitivity cardiac troponin and the Universal Definition of Myocardial Infarction. Circulation 2020;141:161–171.
13. Thygesen K, Jaffe AS. Our nearly complete diagnostic trip of thousands of steps begets a new trip therapeutically. Eur Heart J 2020;41:2217–2219.
14. Lee KK, Shah ASV. High-sensitivity cardiac troponin: a double-edged sword. Eur Heart J Qual Care Clin Outcomes 2020;6:3–4.
15. Kaura A, Sterne JAC, Trickey A, et al. Invasive versus non-invasive management of older patients with non-ST elevation myocardial infarction (SENIOR-NSTEMI): a cohort study based on routine clinical data. Lancet 2020;396:623–634.
16. Bharadwaj A, Potts J, Mohamed MO, et al. Acute myocardial infarction treatments and outcomes in 6.5 million patients with a current or historical diagnosis of cancer in the USA. Eur Heart J 2020;41:2183–2193.
17. Simonsson M, Wallentin L, Alfredsson J, et al. Temporal trends in bleeding events in acute myocardial infarction: insights from the SWEDEHEART registry. Eur Heart J 2020;41: 833–843.
18. Marquis-Gravel G, Dalgaard F, Jones AD, et al. Post-discharge bleeding and mortality following acute coronary syndromes with or without PCI. J Am Coll Cardiol 2020;76:162–171.
19. Ibanez B. And fibrinolysis became pharmaco-invasive. Eur Heart J 2020;41: 855–857.
20. Thrane PG, Kristensen SD, Olesen KKW, et al. 16-year follow-up of the Danish Acute Myocardial Infarction 2 (DANAMI-2) trial: primary percutaneous coronary intervention vs. fibrinolysis in ST-segment elevation myocardial infarction. Eur Heart J 2020;41:847–854.
21. Danchin N, Popovic B, Puymirat E, et al. FAST-MI Investigators. Five-year out comes following timely primary percutaneous intervention, late primary percutaneous intervention, or a pharmaco-invasive strategy in ST-segment elevation myocardial infarction: the FAST-MI programme. Eur Heart J 2020;41:858–866.
22. Zeymer U, Ludman P, Danchin N, et al. The ESC ACCA EAPCI EORP acute coronary syndrome ST-elevation myocardial infarction registry. Eur Heart J Qual Care Clin Outcomes 2020;6:100–104.
23. Scholz KH, Lengenfelder B, Jacobshagen C, et al. Long-term effects of a standardized feedback-driven quality improvement program for timely reperfusion therapy in regional STEMI care networks. Eur Heart J Acute Cardiovasc Care 2020:2048872620907323.
24. Le May M, Wells G, So D, et al. Safety and efficacy of femoral access vs radial access in ST-segment elevation myocardial infarction: the SAFARI-STEMI randomized clinical trial. JAMA Cardiol 2020;5:126–134.
25. Vranckx P, Frigoli E, Rothenbuhler M, et al. MATRIX Investigators. Radial versus femoral access in patients with acute coronary syndromes with or without ST-segment elevation. Eur Heart J 2017;38:1069–1080.
26. Ibanez B, James S, Agewall S, et al. ESC Scientific Document Group. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018;39:119–177.
27. Mehta SR, Wood DA, Storey RF, et al. COMPLETE Trial Steering Committee and Investigators. Complete revascularization with multi-vessel PCI for myocardial infarction. N Engl J Med 2019;381:1411–1421.
28. Bainey KR, Engstrom T, Smits PC, et al. Complete vs culprit-lesion-only revascularization for ST-segment elevation myocardial infarction: a systematic review and meta-analysis. JAMA Cardiol 2020;5:881–888.
29. Atti V, Gwon Y, Narayanan MA, et al. . Multivessel versus culprit-only revascularization in STEMI and multivessel coronary artery disease: meta-analysis of randomized trials. JACC Cardiovasc Interv 2020;13:1571–1582.
30. Sheth T, Pinilla-Echeverri N, Moreno R, et al. Nonculprit lesion severity and outcome of revascularization in patients with STEMI and multivessel coronary disease. J Am Coll Cardiol 2020;76:1277–1286.
31. Piroth Z, Boxma-de Klerk BM, et al. The natural history of nonculprit lesions in STEMI: an FFR substudy of the Compare-Acute Trial. JACC Cardiovasc Interv 2020;13:954–961.
32. Wald DS, Hadyanto S, Bestwick JP. Should fractional flow reserve follow angiographic visual inspection to guide preventive percutaneous coronary intervention in ST-elevation myocardial infarction? Eur Heart J Qual Care Clin Outcomes 2020;6:186–192.
33. Gallone G, Angelini F, Fortuni F, et al. Angiography- vs. physiology-guided complete revascularization in patients with ST-elevation myocardial in- farction and multivessel disease: who is the better gatekeeper in this setting? A meta-analysis of randomized controlled trials. Eur Heart J Qual Care Clin Outcomes 2020;6:199–200.
34. Henderson RA. Fractional flow reserve for non-culprit disease in ST-segment elevation myocardial infarction: first do no harm? Eur Heart J Qual Care Clin Outcomes 2020;6:181–183.
35. Scirica BM, Bergmark BA, Morrow DA, et al. Nonculprit lesion myocardial infarction following percutaneous coronary intervention in patients with acute coronary syndrome. J Am Coll Cardiol 2020;75:1095–1106.
36. Pinilla-Echeverri N, Mehta SR, Wang J, et al. Nonculprit lesion plaque morphology in patients with ST-segment-elevation myocardial infarction: results from the COMPLETE trial optical coherence tomography sub-studies. Circ Cardiovasc Interv 2020;13: e008768.
37. Montone RA, Niccoli G, Crea F, Jang IK. Management of non-culprit coronary plaques in patients with acute coronary syndrome. Eur Heart J 2020;41: 3579–3586.
38. Lorca R, Jimenez-Blanco M, Garcia-Ruiz JM, et al. Coexistence of transmural and lateral wave front progression of myocardial infarction in the human heart. Rev Esp Cardiol (Engl Ed) 2020;
doi: https://doi.org/10.1016/j.rec.2020.07.007
39. Rossello X, Lobo-Gonzalez M, Ibanez B. Editor’s Choice Pathophysiology and therapy of myocardial ischaemia/reperfusion syndrome. Eur Heart J Acute Cardiovasc Care 2019;8:443–456.
40. Ruiz-Meana M, Bou-Teen D, Ferdinandy P, et al. Cardiomyocyte ageing and cardioprotection: consensus document from the ESC working groups cell biology of the heart and myocardial function. Cardiovasc Res 2020;116: 1835–1849.
41. Konijnenberg LSF, Damman P, Duncker DJ, et al. Pathophysiology and diagnosis of coronary microvascular dysfunction in ST-elevation myocardial infarction. Cardiovasc Res 2020;116:787–805.
42. Ibanez B. Intravenous beta-blockers in STEMI: what you are about to do, do it quickly. Eur Heart J Acute Cardiovasc Care 2020;9:459–461.
43. Clemente-Moragon A, Gomez M, Villena-Gutierrez R, et al. Metoprolol exerts a non-class effect against ischaemia-reperfusion injury by abrogating exacerbated inflammation. Eur Heart J 2020;41:4425–4440.
44. Lobo-Gonzalez M, Galan-Arriola C, Rossello X, et al. Metoprolol blunts the time-dependent progression of infarct size. Basic Res Cardiol 2020;115:55.
45. Mathias W Jr, Tsutsui JM, Tavares BG, et al. MRUSMI Investigators. Sonothrombolysis in ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention. J Am Coll Cardiol 2019;73:2832–2842.
46. Aguiar MOD, Tavares BG, Tsutsui JM, et al. Mathias W Jr. Sonothrombolysis improves myocardial dynamics and microvascular obstruction preventing left ventricular remodeling in patients with ST elevation myocardial infarction. Circ Cardiovasc Imaging 2020;13:e009536.
47. Swain L, Reyelt L, Bhave S, et al. Transvalvular ventricular unloading before reperfusion in acute myocardial infarction. J Am Coll Cardiol 2020;76:684–699.
48. Kapur NK, Alkhouli MA, DeMartini TJ, et al. Unloading the left ventricle before reperfusion in patients with anterior ST-segment-elevation myocardial infarction. Circulation 2019;139:337–346.
49. Ibanez B. Intravenous b-blockers in STEMI: what you are about to do, do it quickly. Eur Heart J Acute Cardiovasc Care 2020;
doi: https://doi.org/10.1177/2048872620950205.
50. Lyu J, Wang M, Kang X, et al. Macrophage-mediated regulation of catecholamines in sympathetic neural remodeling after myocardial infarction. Basic Res Cardiol 2020;115:56.
51. Kalla M, Hao G, Tapoulal N, et al. Oxford Acute Myocardial Infarction (OxAMI) Study’, Dall’Armellina E, Banning AP, Choudhury RP, Neubauer S, Kharbanda RK, Channon KM, Ajijola OA, Shivkumar K, Paterson DJ, Herring N. The cardiac sympathetic co-transmitter neuropeptide Y is pro-arrhythmic following ST-elevation myocardial infarction despite beta-blockade. Eur Heart J 2020;41:2168–2179.
52. Kim BK, Hong SJ, Cho YH, et al. TICO Investigators. Effect of ticagre lor monotherapy vs ticagrelor with aspirin on major bleeding and cardiovascular events in patients with acute coronary syndrome: the TICO randomized clinical trial. JAMA 2020;323:2407–2416.
53. Angiolillo DJ, Baber U, Sartori S, et al. Ticagrelor with or without aspirin in high-risk patients with diabetes mellitus undergoing percutaneous coronary intervention. J Am Coll Cardiol 2020;75:2403–2413.
54. Dangas G, Baber U, Sharma S, et al. Ticagrelor with or without aspirin after complex PCI. J Am Coll Cardiol 2020;75:2414–2424.
55. Baber U, Dangas G, Angiolillo DJ, et al. Ticagrelor alone vs. ticagrelor plus aspirin following percutaneous coronary intervention in patients with non-ST-segment elevation acute coronary syndromes: TWILIGHT-ACS. Eur Heart J 2020;41:3533–3545.
56. Hahn JY, Song YB, Oh JH, et al. SMART-CHOICE Investigators. Effect of P2Y12 inhibitor monotherapy vs dual antiplatelet therapy on cardiovascular events in patients undergoing percutaneous coronary intervention: the SMART- CHOICE randomized clinical trial. JAMA 2019;321:2428–2437.
57. Watanabe H, Domei T, Morimoto T, et al. STOPDAPT-2 Investigators. Effect of 1-month dual antiplatelet therapy followed by clopidogrel vs 12-month dual antiplatelet therapy on cardiovascular and bleeding events in patients receiving PCI: the STOPDAPT-2 randomized clinical trial. JAMA 2019;321:2414–2427.
58. Vranckx P, Valgimigli M, Juni P, et al. GLOBAL LEADERS Investigators. Ticagrelor plus aspirin for 1 month, followed by ticagrelor mono therapy for 23 months vs aspirin plus clopidogrel or ticagrelor for 12 months, followed by aspirin monotherapy for 12 months after implantation of a drug eluting stent: a multicentre, open-label, randomised superiority trial. Lancet 2018;392:940–949.
59. O’Donoghue ML, Murphy SA, Sabatine MS. The safety and efficacy of aspirin discontinuation on a background of a P2Y12 inhibitor in patients after percutaneous coronary intervention: a systematic review and meta-analysis. Circulation 2020;142:538–545.
60. D’Ascenzo F, Bertaina M, Fioravanti F, et al. Long versus short dual antiplatelet therapy in acute coronary syndrome patients treated with prasugrel or ticagrelor and coronary revascularization: insights from the RENAMI registry. Eur J Prev Cardiol 2020;27:696–705.
61. Furtado RHM, Nicolau JC, Magnani G, et al. Long-term ticagrelor for secondary prevention in patients with prior myocardial infarction and no history of coronary stenting: insights from PEGASUS-TIMI 54. Eur Heart J 2020;41:1625–1632.
62. Kim HS, Kang J, Hwang D, et al. HOST-REDUCE-POLYTECH- ACS investigators. Prasugrel-based de-escalation of dual antiplatelet therapy after percutaneous coronary intervention in patients with acute coronary syndrome (HOST-REDUCE-POLYTECH-ACS): an open-label, multicentre, non-inferiority randomised trial. Lancet 2020;396:1079–1089.
63. Schupke S, Neumann FJ, Menichelli M, et al. ISAR-REACT 5 Trial Investigators. Ticagrelor or prasugrel in patients with acute coronary syndromes. N Engl J Med 2019;381:1524–1534.
64. Aytekin A, Ndrepepa G, Neumann FJ, et al. Ticagrelor or prasugrel in patients with ST-segment-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Circulation 2020;142:2329–2337.
65. Valina C, Neumann FJ, Menichelli M, et al. Ticagrelor or prasugrel in patients with non-ST-segment elevation acute coronary syndromes. J Am Coll Cardiol 2020;76:2436–2446.
66. Schnorbus B, Daiber A, Jurk K, et al. Effects of clopidogrel vs. prasugrel vs. ticagrelor on endothelial function, inflammatory parameters, and platelet function in patients with acute coronary syndrome undergoing coronary artery stenting: a randomized, blinded, parallel study. Eur Heart J 2020;41:3144–3152.
67. Navarese EP, Khan SU, Kolodziejczak M, et al. Comparative efficacy and safety of oral P2Y12 inhibitors in acute coronary syndrome: network meta-analysis of 52 816 patients from 12 randomized trials. Circulation 2020;142:150–160.
68. You SC, Rho Y, Bikdeli B, et al. Association of ticagrelor vs clopidogrel with net adverse clinical events in patients with acute coronary syndrome undergoing percutaneous coronary intervention. JAMA 2020;324:1640–1650.
69. Tarantini G, Mojoli M, Varbella F, et al. DUBIUS Investigators; Italian Society of Interventional Cardiology. Timing of oral P2Y12 inhibitor administration in non- ST elevation acute coronary syndrome. J Am Coll Cardiol 2020;76:2450–2459.
70. Gargiulo G, Esposito G, Avvedimento M, et al. Cangrelor, tirofiban, and chewed or standard prasugrel regimens in patients with ST-segment-elevation myocardial infarction: primary results of the FABOLUS-FASTER trial. Circulation 2020;142:441–454.
71. Sinnaeve P, Fahrni G, Schelfaut D, et al. Subcutaneous selatogrel inhibits platelet aggregation in patients with acute myocardial infarction. J Am Coll Cardiol 2020;75:2588–2597.
72. Hulot JS, Chevalier B, Belle L, et al. GIANT Investigators. Routine CYP2C19 genotyping to adjust thienopyridine treatment after primary PCI for STEMI: results of the GIANT study. JACC Cardiovasc Interv 2020;13:621–630.
73. Pereira NL, Farkouh ME, So D, et al. Effect of genotype-guided oral P2Y12 inhibitor selection vs conventional clopidogrel therapy on ischemic outcomes after percutaneous coronary intervention: the TAILOR-PCI randomized clinical trial. JAMA 2020;324:761–771.
74. Lewis JP, Backman JD, Reny JL, et al. ICPC Investigators. Pharmacogenomic polygenic response score predicts ischaemic events and cardiovascular mortality in clopidogrel-treated patients. Eur Heart J Cardiovasc Pharmacother 2020;6:203–210.
75. Gimbel M, Qaderdan K, Willemsen L, et al. Clopidogrel ver sus ticagrelor or prasugrel in patients aged 70 years or older with non-ST-elevation acute coronary syndrome (POPular AGE): the randomised, open-label, non-inferiority trial. Lancet 2020;395:1374–1381.
76. Szummer K, Montez-Rath ME, Alfredsson J, et al. Comparison between ticagrelor and clopidogrel in elderly patients with an acute coronary syndrome: insights from the SWEDEHEART registry. Circulation 2020;142:1700–1708.
77. De Filippo O, D’Ascenzo F, Raposeiras-Roubin S, et al. P2Y12 inhibitors in acute coronary syndrome patients with renal dysfunction: an analysis from the RENAMI and BleeMACS projects. Eur Heart J Cardiovasc Pharmacother 2020;6:31–42.
78. Cecconi A, Vilchez-Tschischke JP, Mateo J, et al. Effects of colchicine on atherosclerotic plaque stabilization: a multimodality imaging study in an animal model. J Cardiovasc Transl Res 2020;
doi: https://doi.org/10.1007/s12265-020-09974-7.
79. Tardif JC, Kouz S, Waters DD, et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med 2019;381: 2497–2505.
80. Bouabdallaoui N, Tardif JC, Waters DD, et al. Time-to-treatment initiation of colchicine and cardiovascular outcomes after myocardial infarction in the Colchicine Cardiovascular Outcomes Trial (COLCOT). Eur Heart J 2020; 41: 4092–4099.
81. Shah B, Pillinger M, Zhong H, et al. Effects of acute colchicine administration prior to percutaneous coronary intervention: COLCHICINE-PCI randomized trial. Circ Cardiovasc Interv 2020;13:e008717.
82. Nidorf SM, Fiolet ATL, Mosterd A, et al. LoDoCo2 Trial Investigators. Colchicine in patients with chronic coronary disease. N Engl J Med 2020;383:1838–1847.
83. Tong DC, Quinn S, Nasis A, et al. Colchicine in patients with acute coronary syndrome: the Australian COPS randomized clinical trial. Circulation 2020;142:1890–1900.
84. Kim J, Kang D, Park H, et al. Long-term beta-blocker therapy and clinical outcomes after acute myocardial infarction in patients without heart failure: nationwide cohort study. Eur Heart J 2020;41:3521–3529.
85. Harari R, Bangalore S. Beta-blockers after acute myocardial infarction: an old drug in urgent need of new evidence! Eur Heart J 2020;41:3530–3532.
86. Zeymer U, Bueno H, Granger CB, et al. Acute cardiovascular care association position statement for the diagnosis and treatment of patients with acute myocardial infarction complicated by cardiogenic shock: a document of the Acute Cardiovascular Care Association of the European Society of Cardiology. Eur Heart J Acute Cardiovasc Care 2020;9:183–197.
87. Omer MA, Tyler JM, Henry TD, et al. Clinical characteristics and outcomes of STEMI patients with cardiogenic shock and car diac arrest. JACC Cardiovasc Interv 2020;13:1211–1219.
88. Vallabhajosyula S, Ya’Qoub L, Singh M, et al. Sex disparities in the management and outcomes of cardiogenic shock complicating acute myocardial infarction in the young. Circ Heart Fail 2020;13:e007154.
89. Dhruva SS, Ross JS, Mortazavi BJ, et al. Association of use of an intravascular microaxial left ventricular assist device vs intra-aortic balloon pump with in-hospital mortality and major bleeding among patients with acute myocardial infarction com- plicated by cardiogenic shock. JAMA 2020;323:734–745.
90. Schrage B, Becher PM, Bernhardt A, et al. Left ventricular unloading is associated with lower mortality in patients with cardiogenic shock treated with venoarterial extracorporeal membrane oxygenation: results from an international, multicenter cohort study. Circulation 2020;142:2095–2106.
91. Yannopoulos D, Bartos J, Raveendran G, et al. Advanced reperfusion strategies for patients with out-of-hospital cardiac arrest and refractory ventricular fibrillation (ARREST): a phase 2, single centre, open-label, randomised controlled trial. Lancet 2020;396:1807–1816.
92. Dreyer RP, Tavella R, Curtis JP, et al. Myocardial infarction with non-obstructive coronary arteries as compared with myocardial infarction and obstructive coronary disease: outcomes in a Medicare population. Eur Heart J 2020;41:870–878.
93. Probst S, Seitz A, Martinez Pereyra V, et al. Safety assessment and results of coronary spasm provocation testing in patients with myocardial infarction with unobstructed coronary arteries compared to patients with stable angina and unobstructed coronary arteries. Eur Heart J Acute Cardiovasc Care 2020: 2048872620932422.
94. Gad MM, Mahmoud AN, Saad AM, et al. Incidence, clinical presentation, and causes of 30-day readmission following hospitalization with spontaneous coronary artery dissection. JACC Cardiovasc Interv 2020;13:921–932.
95. Al-Hussaini A, Abdelaty A, Gulsin GS, et al. Chronic infarct size after spontaneous coronary artery dissection: implications for pathophysiology and clinical management. Eur Heart J 2020;41:2197–2205.
96. Ibanez B. [Myocardial infarction in times of COVID-19]. Rev Esp Cardiol 2020; 73:975–977.
97. Chieffo A, Stefanini GG, Price S, et al. EAPCI Position Statement on Invasive Management of Acute Coronary Syndromes during the COVID-19 pandemic. Eur Heart J 2020;41:1839–1851.
98. Rodriguez-Leor O, Cid-Alvarez B, Perez de Prado A, et al. [Impact of COVID-19 on ST-segment elevation myocardial infarction care. The Spanish experience]. Rev Esp Cardiol 2020;73:994–1002.
99. De Rosa S, Spaccarotella C, Basso C, et al. Reduction of hospitalizations for myocardial infarction in Italy in the COVID-19 era. Eur Heart J 2020;41:2083–2088.
100. Metzler B, Siostrzonek P, Binder RK, Bauer A, Reinstadler SJ. Decline of acute coronary syndrome admissions in Austria since the outbreak of COVID-19: the pandemic response causes cardiac collateral damage. Eur Heart J 2020;41: 1852–1853.
101. Mafham MM, Spata E, Goldacre R, et al. COVID-19 pandemic and admission rates for and management of acute coronary syndromes in England. Lancet 2020;396:381–389.
102. Niccoli G, Luescher TF, Crea F. Decreased myocardial infarction admissions during COVID times: what can we learn? Cardiovasc Res 2020;116:e126–e128.
103. Pessoa-Amorim G, Camm CF, Gajendragadkar P, et al. Admission of patients with STEMI since the outbreak of the COVID-19 pandemic: a survey by the European Society of Cardiology. Eur Heart J Qual Care Clin Outcomes 2020;6:210–216.
104. Huber K, Goldstein P. Covid-19: implications for prehospital, emergency and hospital care in patients with acute coronary syndromes. Eur Heart J Acute Cardiovasc Care 2020;9:222–228.
105. Nadkarni GN, Lala A, Bagiella E, et al. Anticoagulation, mortality, bleeding and pathology among patients hospitalized with COVID-19: a single health system study. J Am Coll Cardiol 2020;76:1815–1826.
106. Choudry FA, Hamshere SM, Rathod KS, et al. High thrombus burden in patients with COVID-19 presenting with ST-segment elevation myocardial infarction. J Am Coll Cardiol 2020;76:1168–1176.
107. Solano-Lopez J, Zamorano JL, Pardo Sanz A, et al. [Risk factors for in-hospital mortality in patients with acute myocardial infarction during the COVID-19 outbreak]. Rev Esp Cardiol 2020;73:985–993.
108. Lala A, Johnson KW, Januzzi JL, et al. Mount Sinai COVID Informatics Center. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J Am Coll Cardiol 2020;76:533–546.
109. Puntmann VO, Carerj ML, Wieters I, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020;5:1265–1273.
110. Basso C, Leone O, Rizzo S, et al. Pathological features of COVID-19-associated myocardial injury: a multicentre cardiovascular pathology study. Eur Heart J 2020;41:3827–3835.
111. Frangogiannis NG. The significance of COVID-19-associated myocardial injury: how over-interpretation of scientific findings can fuel media sensationalism and spread misinformation. Eur Heart J 2020;41:3836–3838.
112. Mathur A, Fernandez-Aviles F, Bartunek J, et al. BAMI Group. The effect of intracoronary infusion of bone marrow-derived mononuclear cells on all-cause mortality in acute myocardial infarction: the BAMI trial. Eur Heart J 2020;41:3702–3710.
113. Makkar RR, Kereiakes DJ, Aguirre F, et al. Intracoronary ALLogeneic heart STem cells to Achieve myocardial Regeneration (ALLSTAR): a randomized, placebo-controlled, double-blinded trial. Eur Heart J 2020;41: 3451–3458.